Recent Advances of Chitosan-Based Injectable Hydrogels for Bone and Dental Tissue Regeneration
- PMID: 33042982
- PMCID: PMC7527831
- DOI: 10.3389/fbioe.2020.587658
Recent Advances of Chitosan-Based Injectable Hydrogels for Bone and Dental Tissue Regeneration
Abstract
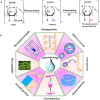
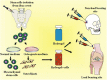
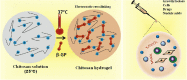
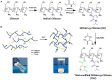
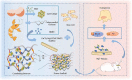
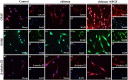
Traditional strategies of bone repair include autografts, allografts and surgical reconstructions, but they may bring about potential hazard of donor site morbidity, rejection, risk of disease transmission and repetitive surgery. Bone tissue engineering (BTE) is a multidisciplinary field that offers promising substitutes in biopharmaceutical applications, and chitosan (CS)-based bone reconstructions can be a potential candidate in regenerative tissue fields owing to its low immunogenicity, biodegradability, bioresorbable features, low-cost and economic nature. Formulations of CS-based injectable hydrogels with thermo/pH-response are advantageous in terms of their high-water imbibing capability, minimal invasiveness, porous networks, and ability to mold perfectly into an irregular defect. Additionally, CS combined with other naturally-derived or synthetic polymers and bioactive agents has proven to be an effective alternative to autologous bone and dental grafts. In this review, we will highlight the current progress in the development of preparation methods, physicochemical properties and applications of CS-based injectable hydrogels and their perspectives in bone and dental regeneration. We believe this review is intended as starting point and inspiration for future research effort to develop the next generation of tissue-engineering scaffold materials.
Keywords: bone repair; chitosan; dental tissue regeneration; injectable hydrogel; responsiveness.
Copyright © 2020 Tang, Tan, Zeng, Wang, Shi, Liu, He, Chen and Ye.
Figures


References
-
- Abdul Rahman N., Nickles K., Gallenbach K., Dannewitz B., Ramich T., Scharf S., et al. (2019). Five-year stability of clinical attachment after regenerative treatment of infrabony defects compared to controls. J. Clin. Periodontol. 46 650–658. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

