Microphysiological Systems: Next Generation Systems for Assessing Toxicity and Therapeutic Effects of Nanomaterials
- PMID: 33043130
- PMCID: PMC7546538
- DOI: 10.1002/smtd.201900589
Microphysiological Systems: Next Generation Systems for Assessing Toxicity and Therapeutic Effects of Nanomaterials
Abstract
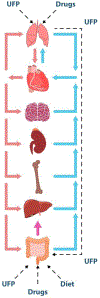
Microphysiological systems, also known as organ-on-a-chip platforms, show promise for the development of new testing methods that can be more accurate than both conventional two-dimensional cultures and costly animal studies. The development of more intricate microphysiological systems can help to better mimic the human physiology and highlight the systemic effects of different drugs and materials. Nanomaterials are among a technologically important class of materials used for diagnostic, therapeutic, and monitoring purposes; all of which and can be tested using new organ-on-a-chip systems. In addition, the toxicity of nanomaterials which have entered the body from ambient air or diet can have deleterious effects on various body systems. This in turn can be studied in newly developed microphysiological systems. While organ-on-a-chip models can be useful, they cannot pick up secondary and systemic toxicity. Thus, the utilization of multi-organ-on-a-chip systems for advancing nanotechnology will largely be reflected in the future of drug development, toxicology studies and precision medicine. Various aspects of related studies, current challenges, and future perspectives are discussed in this paper.
Keywords: multi-organ-on-a-chips; nanomaterials; organ-on-a-chips; personalized medicine; toxicity.
Figures





References
-
- Lin L, Zhao H, Li J, Tang J, Duan M, Jiang L, Biochemical and biophysical research communications 2000, 274, 817. - PubMed
-
- Anonymous, 2018, 191.
-
- Miller MR, Raftis JB, Langrish JP, McLean SG, Samutrtai P, Connell SP, Wilson S, Vesey AT, Fokkens PHB, Boere AJF, Krystek P, Campbell CJ, Hadoke PWF, Donaldson K, Cassee FR, Newby DE, Duffin R, Mills NL, ACS Nano 2017, 11, 4542; - PMC - PubMed
- Järup L, British medical bulletin 2003, 68, 167. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

