Mammalian Expression and In Situ Biotinylation of Extracellular Protein Targets for Directed Evolution
- PMID: 33043224
- PMCID: PMC7542843
- DOI: 10.1021/acsomega.0c03990
Mammalian Expression and In Situ Biotinylation of Extracellular Protein Targets for Directed Evolution
Abstract
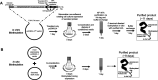
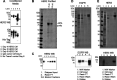
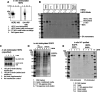
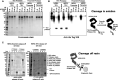
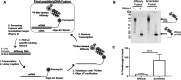
Directed evolution is a powerful tool for the selection of functional ligands from molecular libraries. Extracellular domains (ECDs) of cell surface receptors are common selection targets for therapeutic and imaging agent development. Unfortunately, these proteins are often post-translationally modified and are therefore unsuitable for expression in bacterial systems. Directional immobilization of these targets is further hampered by the absence of biorthogonal groups for site-specific chemical conjugation. We have developed a nonadherent mammalian expression system for rapid, high-yield expression of biotinylated ECDs. ECDs from EGFR, HER2, and HER3 were site-specifically biotinylated in situ and recovered from the cell culture supernatant with yields of up to 10 mg/L at >90% purity. Biotinylated ECDs also contained a protease cleavage site for rapid and selective release of the ECD after immobilization on avidin/streptavidin resins and library binding. A model mRNA display selection round was carried out against the HER2 ECD with the HER2 affibody expressed as an mRNA-protein fusion. HER2 affibody-mRNA fusions were selectively released by thrombin and quantitative PCR revealed substantial improvements in the enrichment of functional affibody-mRNA fusions relative to direct PCR amplification of the resin-bound target. This methodology allows rapid purification of high-quality targets for directed evolution and selective elution of functional sequences at the conclusion of each selection round.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

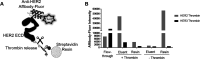



Similar articles
-
An engineered autotransporter-based surface expression vector enables efficient display of Affibody molecules on OmpT-negative E. coli as well as protease-mediated secretion in OmpT-positive strains.Microb Cell Fact. 2014 Dec 30;13:179. doi: 10.1186/s12934-014-0179-z. Microb Cell Fact. 2014. PMID: 25547008 Free PMC article.
-
A plasmid expression system for quantitative in vivo biotinylation of thioredoxin fusion proteins in Escherichia coli.Nucleic Acids Res. 1998 Mar 15;26(6):1414-20. doi: 10.1093/nar/26.6.1414. Nucleic Acids Res. 1998. PMID: 9490786 Free PMC article.
-
Size of the ligand complex between the N-terminal domain of the gene III coat protein and the non-infectious phage strongly influences the usefulness of in vitro selective infective phage technology.Biochem J. 2000 Dec 15;352 Pt 3(Pt 3):841-9. Biochem J. 2000. PMID: 11104694 Free PMC article.
-
Bacterial expression of functional, biotinylated peripheral cannabinoid receptor CB2.Protein Expr Purif. 2006 Sep;49(1):60-70. doi: 10.1016/j.pep.2006.03.002. Epub 2006 Mar 27. Protein Expr Purif. 2006. PMID: 16621595
-
Affinity recovery of eight HER2-binding affibody variants using an anti-idiotypic affibody molecule as capture ligand.Protein Expr Purif. 2011 Mar;76(1):127-35. doi: 10.1016/j.pep.2010.10.008. Epub 2010 Oct 26. Protein Expr Purif. 2011. PMID: 21029777
Cited by
-
Directed Evolution of PD-L1-Targeted Affibodies by mRNA Display.ACS Chem Biol. 2022 Jun 17;17(6):1543-1555. doi: 10.1021/acschembio.2c00218. Epub 2022 May 25. ACS Chem Biol. 2022. PMID: 35611948 Free PMC article.
-
Directing evolution of novel ligands by mRNA display.Chem Soc Rev. 2021 Aug 21;50(16):9055-9103. doi: 10.1039/d1cs00160d. Epub 2021 Jun 24. Chem Soc Rev. 2021. PMID: 34165126 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

