Bioengineering studies and pathway modeling of the heterologous biosynthesis of tetrahydrocannabinolic acid in yeast
- PMID: 33043390
- PMCID: PMC7595985
- DOI: 10.1007/s00253-020-10798-3
Bioengineering studies and pathway modeling of the heterologous biosynthesis of tetrahydrocannabinolic acid in yeast
Abstract
Heterologous biosynthesis of tetrahydrocannabinolic acid (THCA) in yeast is a biotechnological process in Natural Product Biotechnology that was recently introduced. Based on heterologous genes from Cannabis sativa and Streptomyces spp. cloned into Saccharomyces cerevisiae, the heterologous biosynthesis was fully embedded as a proof of concept. Low titer and insufficient biocatalytic rate of most enzymes require systematic optimization of recombinant catalyst by protein engineering and consequent C-flux improvement of the yeast chassis for sufficient precursor (acetyl-CoA), energy (ATP), and NADH delivery. In this review basic principles of in silico analysis of anabolic pathways towards olivetolic acid (OA) and cannabigerolic acid (CBGA) are elucidated and discussed to identify metabolic bottlenecks. Based on own experimental results, yeasts are discussed as potential platform organisms to be introduced as potential cannabinoid biofactories. Especially feeding strategies and limitations in the committed mevalonate and olivetolic acid pathways are in focus of in silico and experimental studies to validate the scientific and commercial potential as a realistic alternative to the plant Cannabis sativa.Key points• First time critical review of the heterologous process for recombinant THCA/CBDA production and critical review of bottlenecks and limitations for a bioengineered technical process• Integrative approach of protein engineering, systems biotechnology, and biochemistry of yeast physiology and biosynthetic cannabinoid enzymes• Comparison of NphB and CsPT aromatic prenyltransferases as rate-limiting catalytic steps towards cannabinoids in yeast as platform organisms Graphical abstract.
Keywords: Bioengineering; Cannabidiol; Cannabinoids; Cannabis sativa; CsPT; Natural Product Biotechnology; NphB; Synthetic biology; Tetrahydrocannabinol.
Conflict of interest statement
OK declares that he has no conflict of interest or any competing interests. CS declares that she has no conflict of interest or any competing interests. FT declares that he has no conflict of interest or any competing interests. OK declares that he is listed as an inventor on the patent DE 102018117233 A1 issued on January 23, 2020.
Figures

 ), hexanoyl-CoA (HCoA,
), hexanoyl-CoA (HCoA,  ), and olivetolic acid (OA,
), and olivetolic acid (OA,  ) reach an intracellular non-toxic steady state
) reach an intracellular non-toxic steady state
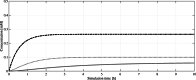
 ), geranyl pyrophosphate (GPP,
), geranyl pyrophosphate (GPP,  ), and (CBGA,
), and (CBGA,  ) over a simulation time of 10 h. After several genetic optimizations and in contrast to the accumulation of GPP, OA reaches a stable equilibrium concentration. This implies that it is the limiting intermediate in the subsequent formation of CBGA
) over a simulation time of 10 h. After several genetic optimizations and in contrast to the accumulation of GPP, OA reaches a stable equilibrium concentration. This implies that it is the limiting intermediate in the subsequent formation of CBGA
 ) and geranyl pyrophosphate (GPP,
) and geranyl pyrophosphate (GPP,  ) accumulate, cannabigerolic acid (CBGA,
) accumulate, cannabigerolic acid (CBGA,  ) reaches a steady state while being used as substrate itself. Neither isopentenyl pyrophosphate (IPP,
) reaches a steady state while being used as substrate itself. Neither isopentenyl pyrophosphate (IPP,  ) nor dimethylallyl pyrophosphate (DMAPP,
) nor dimethylallyl pyrophosphate (DMAPP,  ) are detectable in significant amounts as they get converted into GPP/FPP efficiently
) are detectable in significant amounts as they get converted into GPP/FPP efficiently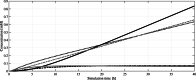
 ) and olivetolic acid (OA,
) and olivetolic acid (OA,  ), cannabigerolic acid (CBGA,
), cannabigerolic acid (CBGA,  ) is formed, which itself is converted to the product Δ9-tetrahydrocannabinolic acid (THCA,
) is formed, which itself is converted to the product Δ9-tetrahydrocannabinolic acid (THCA,  ). The remaining farnesyl pyrophosphate (FPP,
). The remaining farnesyl pyrophosphate (FPP,  ) concentration can be seen as potential to increase GPP production in future attempts
) concentration can be seen as potential to increase GPP production in future attempts
References
-
- Asakawa Y, Hashimoto T, Takikawa K, Tori M, Ogawa S. Prenyl bibenzyls from the liverworts Radula perrottetii and Radula complanata. Phytochemistry. 1991;30:235–251. doi: 10.1016/0031-9422(91)84130-K. - DOI
-
- Brenda (2019) No Title. https://www.brenda-enzymes.info/. Accessed 14 Nov 2019
-
- Chen N, Koumpouras GC, Polizzi KM, Kontoravdi C (2012) Genome-based kinetic modeling of cytosolic glucose metabolism in industrially relevant cell lines: Saccharomyces cerevisiae and Chinese hamster ovary cells. Bioprocess Biosyst Eng 35:1023–1033. 10.1007/s00449-012-0687-3 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

