Trimers Conjugated to Fibrin Hydrogels Promote Salivary Gland Function
- PMID: 33043768
- PMCID: PMC7903845
- DOI: 10.1177/0022034520964784
Trimers Conjugated to Fibrin Hydrogels Promote Salivary Gland Function
Abstract
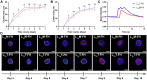
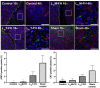
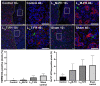
New strategies for tissue engineering have great potential for restoring and revitalizing impaired tissues and organs, including the use of smart hydrogels that can be modified to enhance organization and functionality of the salivary glands. For instance, monomers of laminin-111 peptides chemically conjugated to fibrin hydrogel (L1pM-FH) promote cell cluster formation in vitro and salivary gland regeneration in vivo when compared with fibrin hydrogel (FH) alone; however, L1pM-FH produce only weak expression of acinar differentiation markers in vivo (e.g., aquaporin-5 and transmembrane protein 16). Since previous studies demonstrated that a greater impact can be achieved when trimeric forms were used as compared with monomeric or dimeric forms, we investigated the extent to which trimers of laminin-111 chemically conjugated to FH (L1pT-FH) can increase the expression of acinar differentiation markers and elevate saliva secretion. In vitro studies using Par-C10 acinar cells demonstrated that when compared with L1pM-FH, L1pT-FH induced similar levels of acinar-like cell clustering, polarization, lumen formation, and calcium signaling. To assess the performance of the trimeric complex in vivo, we compared the ability of L1pM-FH and L1pT-FH to increase acinar differentiation markers and restore saliva flow rate in a salivary gland wound model of C57BL/6 mice. Our results show that L1pT-FH applied to wounded mice significantly improved the expression of the acinar differentiation markers and saliva secretion when compared with the monomeric form. Together, these positive effects of L1pT-FH warrant its future testing in additional models of hyposalivation with the ultimate goal of applying this technology in humans.
Keywords: biocompatibility; bioengineering; biomaterial(s); cell-matrix interactions; regeneration; saliva.
Conflict of interest statement
Figures


References
-
- Almstahl A, Skoogh Andersson J, Alstad T, Fagerberg-Mohlin B, Finizia C. 2019. Explorative study on quality of life in relation to salivary secretion rate in head and neck cancer patients treated with radiotherapy up to 2 years post treatment. Int J Dent Hygiene. 17(1):46–54. - PubMed
-
- Baker OJ, Camden JM, Redman RS, Jones JE, Seye CI, Erb L, Weisman GA. 2008. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma alter tight junction structure and function in the rat parotid gland Par-C10 cell line. Am J Physiol Cell Physiol. 295(5):C1191–C1201. - PMC - PubMed
-
- Boonyapranai K, Tsai HY, Chen MC, Sriyam S, Sinchaikul S, Phutrakul S, Chen ST. 2011. Glycoproteomic analysis and molecular modeling of haptoglobin multimers. Electrophoresis. 32(12):1422–1432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

