Validation and clinical evaluation of a SARS-CoV-2 surrogate virus neutralisation test (sVNT)
- PMID: 33043818
- PMCID: PMC7605318
- DOI: 10.1080/22221751.2020.1835448
Validation and clinical evaluation of a SARS-CoV-2 surrogate virus neutralisation test (sVNT)
Abstract
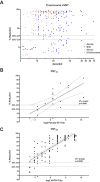
To understand SARS-CoV-2 immunity after natural infection or vaccination, functional assays such as virus neutralising assays are needed. So far, assays to detect SARS-CoV-2 neutralising antibodies rely on cell-culture based infection assays either using wild type SARS-CoV-2 or pseudotyped viruses. Such assays are labour-intensive, require appropriate biosafety facilities and are difficult to standardize. Recently, a new surrogate virus neutralisation test (sVNT) was described that uses the principle of an ELISA to measure the neutralisation capacity of anti-SARS-CoV-2 antibodies directed against the receptor binding domain. Here, we performed an independent evaluation of the robustness, specificity and sensitivity on an extensive panel of sera from 269 PCR-confirmed COVID-19 cases and 259 unmatched samples collected before 2020 and compared it to cell-based neutralisation assays. We found a high specificity of 99.2 (95%CI: 96.9-99.9) and overall sensitivity of 80.3 (95%CI: 74.9-84.8) for the sVNT. Clinical sensitivity increased between early (<14 days post symptom onset or post diagnosis, dpos/dpd) and late sera (>14 dpos/dpd) from 75.0 (64.7-83.2) to 83.1 (76.5-88.1). Also, higher severity was associated with an increase in clinical sensitivity. Upon comparison with cell-based neutralisation assays we determined an analytical sensitivity of 74.3 (56.4-86.9) and 98.2 (89.4-99.9) for titres ≥10 to <40 and ≥40 to <160, respectively. Only samples with a titre ≥160 were always positive in the sVNT. In conclusion, the sVNT can be used as an additional assay to determine the immune status of COVID-19 infected of vaccinated individuals but its value needs to be assessed for each specific context.
Keywords: SARS-CoV-2; cell-based virus neutralisation assay; neutralising antibodies; pseudovirus neutralisation assay; surrogate virus neutralisation assay.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- Foundation for Innovative New Diagnostics . FIND evaluation update: SARS-CoV-2 immunoassays. FIND evaluation update: SARS-CoV-2 immunoassays https://www.finddx.org/covid-19/sarscov2-eval-immuno/ (2020).
-
- Lassaunière R, Frische A, Harboe ZB, AC Nielsen, Fomsgaard A, KA Krogfelt, Jørgensen CS.. Evaluation of nine commercial SARS-CoV-2 immunoassays. medRxiv. 2020. doi:10.1101/2020.04.09.20056325 - DOI
-
- GeurtsvanKessel CH, NMA Okba, Igloi Z, Bogers S, CWE Embregts, BM Laksono, Leijten L, Rokx C, Rijnders B, Rahamat-Langendoen J, et al. . An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat Commun. 2020;11:3436. doi:10.1038/s41467-020-17317-y - DOI - PMC - PubMed
-
- Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor-Phillips S, Adriano A, Beese S, Dretzke J, di Ruffano L Ferrante, et al. . Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020;(6):Art. No. CD013652. doi:10.1002/14651858.CD013652 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
