Rapid purification and metabolomic profiling of synaptic vesicles from mammalian brain
- PMID: 33043885
- PMCID: PMC7575323
- DOI: 10.7554/eLife.59699
Rapid purification and metabolomic profiling of synaptic vesicles from mammalian brain
Abstract
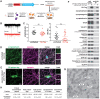
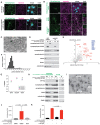
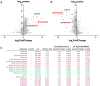
Neurons communicate by the activity-dependent release of small-molecule neurotransmitters packaged into synaptic vesicles (SVs). Although many molecules have been identified as neurotransmitters, technical limitations have precluded a full metabolomic analysis of SV content. Here, we present a workflow to rapidly isolate SVs and to interrogate their metabolic contents at high-resolution using mass spectrometry. We validated the enrichment of glutamate in SVs of primary cortical neurons using targeted polar metabolomics. Unbiased and extensive global profiling of SVs isolated from these neurons revealed that the only detectable polar metabolites they contain are the established neurotransmitters glutamate and GABA. In addition, we adapted the approach to enable quick capture of SVs directly from brain tissue and determined the neurotransmitter profiles of diverse brain regions in a cell-type-specific manner. The speed, robustness, and precision of this method to interrogate SV contents will facilitate novel insights into the chemical basis of neurotransmission.
Keywords: metabolomics; mouse; neuroscience; neurotransmitters; synaptic vesicle.
© 2020, Chantranupong et al.
Conflict of interest statement
LC, JS, WW, DJ, MP, BS No competing interests declared
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

