Comparison of Parental Report of Influenza Vaccination to Documented Records in Children Hospitalized With Acute Respiratory Illness, 2015-2016
- PMID: 33043965
- PMCID: PMC9264279
- DOI: 10.1093/jpids/piaa110
Comparison of Parental Report of Influenza Vaccination to Documented Records in Children Hospitalized With Acute Respiratory Illness, 2015-2016
Abstract
Background: Parent-reported influenza vaccination history may be valuable clinically and in influenza vaccine effectiveness (VE) studies. Few studies have assessed the validity of parental report among hospitalized children.
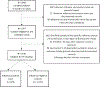
Methods: Parents of 2597 hospitalized children 6 months-17 years old were interviewed from November 1, 2015 to June 30, 2016, regarding their child's sociodemographic and influenza vaccination history. Parent-reported 2015-2016 influenza vaccination history was compared with documented vaccination records (considered the gold standard for analysis) obtained from medical records, immunization information systems, and providers. Multivariable logistic regression analyses were conducted to determine potential factors associated with discordance between the 2 sources of vaccination history. Using a test-negative design, we estimated VE using vaccination history obtained through parental report and documented records.
Results: According to parental report, 1718 (66%) children received the 2015-2016 influenza vaccine, and of those, 1432 (83%) had documentation of vaccine receipt. Percent agreement was 87%, with a sensitivity of 96% (95% confidence interval [CI], 95%-97%) and a specificity of 74% (95% CI, 72%-77%). In the multivariable logistic regression, study site and child's age 5-8 years were significant predictors of discordance. Adjusted VE among children who received ≥1 dose of the 2015-2016 influenza vaccine per parental report was 61% (95% CI, 43%-74%), whereas VE using documented records was 55% (95% CI, 33%-69%).
Conclusions: Parental report of influenza vaccination was sensitive but not as specific compared with documented records. However, VE against influenza-associated hospitalizations using either source of vaccination history did not differ substantially. Parental report is valuable for timely influenza VE studies.
Keywords: discordance; immunization record; influenza vaccination; parental report; vaccine effectiveness; validity.
Published by Oxford University Press on behalf of The Journal of the Pediatric Infectious Diseases Society 2020.
Figures
References
-
- Chaves SS, Perez A, Farley MM, et al. ; Influenza Hospitalization Surveillance Network. The burden of influenza hospitalizations in infants from 2003 to 2012, United States. Pediatr Infect Dis J 2014; 33:912–9. - PubMed
-
- Chung JR, Rolfes MA, Flannery B, et al. Effects of influenza vaccination in the United States during the 2018–2019 influenza season [manuscript published online ahead of print 6 January 2020]. Clin Infect Dis 2020. doi: 10.1093/cid/ciz1244. - DOI
-
- Fiore AE, Shay DK, Broder K, et al. ; Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep 2008; 57:1–60. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical