Mucosal glycan degradation of the host by the gut microbiota
- PMID: 33043970
- PMCID: PMC8252862
- DOI: 10.1093/glycob/cwaa097
Mucosal glycan degradation of the host by the gut microbiota
Abstract
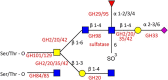
The gut microbiota plays a major role in human health and an alteration in gut microbiota structure and function has been implicated in several diseases. In the colon, mucus covering the epithelium is critical to maintain a homeostatic relationship with the gut microbiota by harboring a microbial community at safe distance from the epithelium surface. The mucin glycans composing the mucus layer provide binding sites and a sustainable source of nutrients to the bacteria inhabiting the mucus niche. Access to these glycan chains requires a complement of glycoside hydrolases (GHs) produced by bacteria across the phyla constituting the human gut microbiota. Due to the increased recognition of the role of mucus-associated microbes in human health, how commensal bacteria breakdown and utilize host mucin glycans has become of increased interest and is reviewed here. This short review provides an overview of the strategies evolved by gut commensal bacteria to access this rich source of the nutrient with a focus on the GHs involved in mucin degradation.
Keywords: glycosylation; gut microbiota; mucin; mucus.
© The Author(s) 2020. Published by Oxford University Press.
Figures
References
-
- Ashida H, Miyake A, Kiyohara M, Wada J, Yoshida E, Kumagai H, Katayama T, Yamamoto K. 2009. Two distinct Α-L-Fucosidases from Bifidobacterium Bifidum are essential for the utilization of Fucosylated milk oligosaccharides and Glycoconjugates. Glycobiology. 19:1010–1017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/F/00044452/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/R012490/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/F/000PR10353 /BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/P008895/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/M011216/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases