Hydroxychloroquine in Hospitalized Patients with COVID-19: Real-World Experience Assessing Mortality
- PMID: 33044019
- PMCID: PMC7675747
- DOI: 10.1002/phar.2467
Hydroxychloroquine in Hospitalized Patients with COVID-19: Real-World Experience Assessing Mortality
Abstract
Introduction: Hydroxychloroquine (HCQ) for coronavirus disease 2019 (COVID-19) is presently being used off-label or within a clinical trial.
Objectives: We investigated a multinational database of patients with COVID-19 with real-world data containing outcomes and their relationship to HCQ use. The primary outcome was all-cause mortality within 30 days of follow-up.
Methods: This was a retrospective cohort study of patients receiving HCQ within 48 hours of hospital admission. Medications, preexisting conditions, clinical measures on admission, and outcomes were recorded.
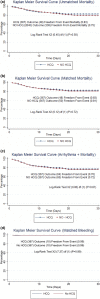
Results: Among patients with a diagnosis of COVID-19 in our propensity-matched cohort, the mean ages ± SD were 62.3 ± 15.9 years (53.7% male) and 61.9 ± 16.0 years (53.0% male) in the HCQ and no-HCQ groups, respectively. There was no difference in overall 30-day mortality between the HCQ and no-HCQ groups (HCQ 13.1%, n=367; no HCQ 13.6%, n=367; odds ratio 0.95, 95% confidence interval 0.62-1.46) after propensity matching. Although statistically insignificant, the HCQ-azithromycin (AZ) group had an overall mortality rate of 14.6% (n=199) compared with propensity-matched no-HCQ-AZ cohort's rate of 12.1% (n=199, OR 1.24, 95% CI 0.70-2.22). Importantly, however, there was no trend in this cohort's overall mortality/arrhythmogenesis outcome (HCQ-AZ 17.1%, no HCQ-no AZ 17.1%; OR 1.0, 95% CI 0.6-1.7).
Conclusions: We report from a large retrospective multinational database analysis of COVID-19 outcomes with HCQ and overall mortality in hospitalized patients. There was no statistically significant increase in mortality and mortality-arrhythmia with HCQ or HCQ-AZ.
Keywords: Antimalarial; azithromycin; coronavirus; hydroxychloroquine; macrolide; severe acute respiratory syndrome coronavirus 2.
© 2020 Pharmacotherapy Publications, Inc.
Figures

References
-
- Coronavirus (COVID‐19) update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine [press release], 2020.
-
- Kapoor R. Pharmacovigilance Memorandum Department of Health and Human Services 2020. Available from https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/OSE%20Review_Hyd.... Accessed November 2, 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

