Simvastatin Treatment Does Not Ameliorate Muscle Pathophysiology in a Mouse Model for Duchenne Muscular Dystrophy
- PMID: 33044191
- PMCID: PMC8543260
- DOI: 10.3233/JND-200524
Simvastatin Treatment Does Not Ameliorate Muscle Pathophysiology in a Mouse Model for Duchenne Muscular Dystrophy
Abstract
Duchenne muscular dystrophy is an X-linked, recessive muscular dystrophy in which the absence of the dystrophin protein leads to fibrosis, inflammation and oxidative stress, resulting in loss of muscle tissue. Drug repurposing, i.e. using drugs already approved for other disorders, is attractive as it decreases development time. Recent studies suggested that simvastatin, a cholesterol lowering drug used for cardiovascular diseases, has beneficial effects on several parameters in mdx mice. To validate properly the effectiveness of simvastatin, two independent labs tested the effects of 12-week simvastatin treatment in either young (starting at 4 weeks of age) or adult (starting at 12 weeks of age) mdx mice. In neither study were benefits of simvastatin treatment observed on muscle function, histology or expression of genes involved in fibrosis, regeneration, oxidative stress and autophagy. Unexpectedly, although the treatment protocol was similar, simvastatin plasma levels were found to be much lower than observed in a previous study. In conclusion, in two laboratories, simvastatin did not ameliorate disease pathology in mdx mice, which could either be due to the ineffectiveness of simvastatin itself or due to the low simvastatin plasma levels following oral administration via the food.
Conflict of interest statement
IV, OC, CTdW, JJPs, SN, KW and JH have no conflict of interest to report. DB is research director of Duchenne UK. AAR discloses being employed by LUMC which has patents on exon skipping technology, some of which has been licensed to BioMarin and subsequently sublicensed to Sarepta. As co-inventor of some of these patents AAR is entitled to a share of royalties. AAR reports that she is on the scientific advisory board of Duchenne UK, but excused herself from commenting on this application when it was submitted for evaluation. AAR further discloses being ad hoc consultant for PTC Therapeutics, Sarepta Therapeutics, CRISPR Therapeutics, Summit PLC, Alpha Anomeric, BioMarin Pharmaceuticals Inc., Eisai, Astra Zeneca, Santhera, Audentes, Global Guidepoint and GLG consultancy, Grunenthal, Wave and BioClinica, having been a member of the Duchenne Network Steering Committee (BioMarin) and being a member of the scientific advisory boards of ProQR, hybridize therapeutics, silence therapeutics, Sarepta therapeutics and Philae Pharmaceuticals. Remuneration for these activities is paid to LUMC. LUMC also received speaker honoraria from PTC Therapeutics and BioMarin Pharmaceuticals and funding for contract research from Italfarmaco and Alpha Anomeric. Project funding is received from Sarepta Therapeutics. DJW has been an ad hoc consultant for a large number of companies and is on the Scientific Advisory Board for Akashi Therapeutics. Studies in the Wells laboratory have been funded by Proximagen and Shire Pharmaceuticals.
Figures




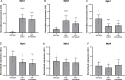




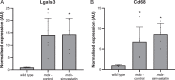
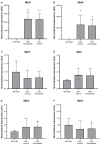



References
-
- Mendell JR, Lloyd-Puryear M. Report of MDA muscle disease symposium on newborn screening for Duchenne muscular dystrophy. Muscle Nerve. 2013;48(1):21–6. - PubMed
-
- Emery AEH, Muntoni F, Quinlivan RCM. Duchenne muscular dystrophy. 4th ed: Oxford University Press; 2015May 2015.
-
- Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919–28. - PubMed
-
- Koenig M, Monaco AP, Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53(2):219–28. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

