Global immunoglobulin supply: steaming towards the iceberg?
- PMID: 33044340
- PMCID: PMC7752222
- DOI: 10.1097/ACI.0000000000000696
Global immunoglobulin supply: steaming towards the iceberg?
Abstract
Purpose of review: This review describes how plasma is sourced for fractionation into plasma-derived medicinal products (PDMPs), such as immunoglobulin (Ig) together with differences between plasma from whole blood (recovered plasma) and from plasmapheresis (source plasma) in terms of global plasma supply. Specific areas of growth in immunoglobulin use are identified alongside novel therapies, which may reduce demand for some immunoglobulin indications.
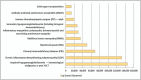
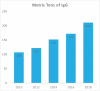
Recent findings: There has been a 6--8% annual growth in immunoglobulin use. Secondary immunodeficiency alongside improved recognition and diagnosis primary immunodeficiency disorders are drivers whereas the novel neonatal Fc receptor inhibitors (FcRni) may reduce demand for some immunomodulatory indications.
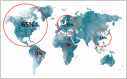
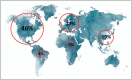
Summary: There is a significant geographical imbalance in global supply of plasma with 65% collected in the United States. This results in a dependency of other countries on United States supply and argues for both more plasma supply and greater regionally balanced plasma collection. In addition, progress towards a transparent, regulated and well tolerated framework for the coexistence of unpaid and compensated plasma donations is needed as unpaid donation will not be sufficient. These discussions should be informed by the needs of patients for this life-saving therapy, the care of donors and the safety of plasma and PDMPs.
Conflict of interest statement
S.J. declares Advisory Board, Speaker, Conference, Clinical Trial, DSMB, or Projects with CSL Behring, Shire, Takeda, Thermofisher, Swedish Orphan Biovitrum, Biotest, Binding Site, BPL, Octapharma, Sanofi, LFB, Pharming, Biocryst, Zarodex, Weatherden and UCB Pharma.
Figures

Similar articles
-
Review of indications for immunoglobulin (IG) use: Narrowing the gap between supply and demand.Transfus Clin Biol. 2021 Feb;28(1):96-122. doi: 10.1016/j.tracli.2020.12.005. Epub 2020 Dec 13. Transfus Clin Biol. 2021. PMID: 33321210 Review.
-
The growing importance of achieving national self-sufficiency in immunoglobulin in Italy. The emergence of a national imperative.Blood Transfus. 2019 Nov;17(6):449-458. doi: 10.2450/2019.0265-19. Epub 2019 Dec 11. Blood Transfus. 2019. PMID: 31846609 Free PMC article. Review.
-
Estimation of the latent therapeutic demand for immunoglobulin therapies in autoimmune neuropathies in the United States.Vox Sang. 2022 Feb;117(2):208-219. doi: 10.1111/vox.13134. Epub 2021 Jun 10. Vox Sang. 2022. PMID: 34110626
-
Shortage of plasma-derived medicinal products: what is next? narrative literature review on its causes and counteracting policies in Italy.Front Pharmacol. 2024 May 6;15:1375891. doi: 10.3389/fphar.2024.1375891. eCollection 2024. Front Pharmacol. 2024. PMID: 38769998 Free PMC article. Review.
-
Update on the use of immunoglobulin in human disease: A review of evidence.J Allergy Clin Immunol. 2017 Mar;139(3S):S1-S46. doi: 10.1016/j.jaci.2016.09.023. Epub 2016 Dec 29. J Allergy Clin Immunol. 2017. PMID: 28041678 Review.
Cited by
-
A homogeneous bioluminescent immunoassay for parallel characterization of binding between a panel of antibodies and a family of Fcγ receptors.Sci Rep. 2022 Jul 16;12(1):12185. doi: 10.1038/s41598-022-15887-z. Sci Rep. 2022. PMID: 35842448 Free PMC article.
-
Using a scenario approach to assess for the current and future demand of immunoglobulins: An interview and literature study from The Netherlands.Transfus Med. 2022 Oct;32(5):410-421. doi: 10.1111/tme.12889. Epub 2022 Jun 24. Transfus Med. 2022. PMID: 35751376 Free PMC article. Review.
-
Effectiveness of immunoglobulin replacement therapy in preventing infections in patients with chronic obstructive pulmonary disease: a systematic review.Allergy Asthma Clin Immunol. 2024 Apr 10;20(1):30. doi: 10.1186/s13223-024-00886-8. Allergy Asthma Clin Immunol. 2024. PMID: 38600554 Free PMC article.
-
SARS-CoV-2 booster vaccination rescues attenuated IgG1 memory B cell response in primary antibody deficiency patients.Front Immunol. 2022 Dec 22;13:1033770. doi: 10.3389/fimmu.2022.1033770. eCollection 2022. Front Immunol. 2022. PMID: 36618402 Free PMC article.
-
Access and use of immunoglobulins in supportive cancer care: A thematic analysis of a systematic review data set.J Med Access. 2024 Mar 28;8:27550834241236596. doi: 10.1177/27550834241236596. eCollection 2024 Jan-Dec. J Med Access. 2024. PMID: 38559466 Free PMC article.
References
-
- Bruton OC. Agammaglobulinemia. Pediatrics 1952; 9:722–728. - PubMed
-
- European Medicines Agency. Guideline on the clinical investigation of human normal immunoglobulin for intravenous administration (IVIg). EMA/CHMP/BPWP/94033/2007 rev. 3. Committee for Medicinal Products for Human Use (CHMP), 28 June 2018. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-cl... (Accessed 15 June 2019)
-
- European Medicines Agency. Guideline on the clinical investigation of human normal immunoglobulin for subcutaneous and/or intramuscular administration (SCIg/IMIg). EMA/CHMP/BPWP/410415/2011 rev 1. 23 July, 2015. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-cl... (Accessed 15 June 2020)
-
- European Medicines Agency. Guideline on plasma-derived medicinal products. EMA/CHMP/BWP/706271/2010. London. 21 July, 2011. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-pl... (Accessed 29 April 2019)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

