The Parkinson disease pain classification system: results from an international mechanism-based classification approach
- PMID: 33044395
- PMCID: PMC7977616
- DOI: 10.1097/j.pain.0000000000002107
The Parkinson disease pain classification system: results from an international mechanism-based classification approach
Abstract
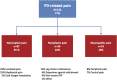
Pain is a common nonmotor symptom in patients with Parkinson disease (PD) but the correct diagnosis of the respective cause remains difficult because suitable tools are lacking, so far. We developed a framework to differentiate PD- from non-PD-related pain and classify PD-related pain into 3 groups based on validated mechanistic pain descriptors (nociceptive, neuropathic, or nociplastic), which encompass all the previously described PD pain types. Severity of PD-related pain syndromes was scored by ratings of intensity, frequency, and interference with daily living activities. The PD-Pain Classification System (PD-PCS) was compared with classic pain measures (ie, brief pain inventory and McGill pain questionnaire [MPQ], PDQ-8 quality of life score, MDS-UPDRS scores, and nonmotor symptoms). 159 nondemented PD patients (disease duration 10.2 ± 7.6 years) and 37 healthy controls were recruited in 4 centers. PD-related pain was present in 122 patients (77%), with 24 (15%) suffering one or more syndromes at the same time. PD-related nociceptive, neuropathic, or nociplastic pain was diagnosed in 87 (55%), 25 (16%), or 35 (22%), respectively. Pain unrelated to PD was present in 35 (22%) patients. Overall, PD-PCS severity score significantly correlated with pain's Brief Pain Inventory and MPQ ratings, presence of dyskinesia and motor fluctuations, PDQ-8 scores, depression, and anxiety measures. Moderate intrarater and interrater reliability was observed. The PD-PCS is a valid and reliable tool for differentiating PD-related pain from PD-unrelated pain. It detects and scores mechanistic pain subtypes in a pragmatic and treatment-oriented approach, unifying previous classifications of PD-pain.
Copyright © 2020 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association for the Study of Pain.
Conflict of interest statement
V. Mylius received honoraria from LicherMT, Boston Scientific, and Abbvie. SPL received honoraria from IPMDS and consulted for Merz Pharmaceuticals. F. Brugger received research grants from Baasch-Medicus Foundation and Forschungskommission Kantonsspital St. Gallen, and a travel grant from Abbvie Switzerland. A. Rizos received salary support from the Institute for National Health Research (NIHR) Clinical Research Network (CRN) South London and honoraria from Britannia Pharmaceuticals Ltd. K.R. Chaudhuri received honoraria for advisory boards by AbbVie, UCB, Pfizer, Jazz Pharma, GKC, Bial, Cynapsus, Novartis, Lobsor, Stada, Medtronic, Zambon, Profile, Sunovion, Roche, Therevance, and Scion, honoraria for lectures by AbbVie, Britannia, UCB, Mundipharma, Zambon, Novartis, Boeringer Ingelheim, Neuroderm, and Sunovion, grants (investigator-initiated) by Britania Pharmaceuticals, AbbVie, UCB, GKC, and Bial, academic grants by EU (Horizon 2020), IMI, Parkinson UK, NIHR, PDNMG, Kirby Laing Foundation, NPF, and MRC. L. Timmermann received payments as a consultant for Boston Scientific, and honoraria as a speaker on symposia sponsored by UCB, Desitin, Boston Scientific, and Abbott. R. Gonzenbach received honoraria from Almirall, Bayer, Sandoz, Biogen, Roche, Novartis, and Sanofi. G. Kägi received honoraria from Bayer. D. Ciampi de Andrade consulted for Merk, Grunenthal, Cristalia, and Novartis, and investigator-initiated research grant from Grunenthal, Mundipharma, Pfizer, and Abbott. The remaining authors have no conflicts of interest to declare.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures

References
-
- Attal N, Fermanian C, Fermanian J, Lanteri-Minet M, Alchaar H, Bouhassira D. Neuropathic pain: are there distinct subtypes depending on the aetiology or anatomical lesion? PAIN 2008;138:343–53. - PubMed
-
- Aziz Q, Giamberardino MA, Barke A, Korwisi B, Baranowski AP, Wesselmann U, Rief W, Treede RD, Pain ITftCoC. The IASP classification of chronic pain for ICD-11: chronic secondary visceral pain. PAIN 2019;160:69–76. - PubMed
-
- Beiske AG, Loge JH, Ronningen A, Svensson E. Pain in Parkinson's disease: prevalence and characteristics. PAIN 2009;141:173–7. - PubMed
-
- Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, Cunin G, Fermanian J, Ginies P, Grun-Overdyking A, Jafari-Schluep H, Lanteri-Minet M, Laurent B, Mick G, Serrie A, Valade D, Vicaut E. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). PAIN 2005;114:29–36. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

