Ultra-High-Speed Western Blot using Immunoreaction Enhancing Technology
- PMID: 33044451
- PMCID: PMC8504990
- DOI: 10.3791/61657
Ultra-High-Speed Western Blot using Immunoreaction Enhancing Technology
Abstract
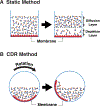
A western blot (also known as an immunoblot) is a canonical method for biomedical research. It is commonly used to determine the relative size and abundance of specific proteins as well as post-translational protein modifications. This technique has a rich history and remains in widespread use due to its simplicity. However, the western blotting procedure famously takes hours, even days, to complete, with a critical bottleneck being the long incubation times that limit its throughput. These incubation steps are required due to the slow diffusion of antibodies from the bulk solution to the immobilized antigens on the membrane: the antibody concentration near the membrane is much lower than the bulk concentration. Here, we present an innovation that dramatically reduces these incubation intervals by improving antigen binding via cyclic draining and replenishing (CDR) of the antibody solution. We also utilized an immunoreaction enhancing technology to preserve the sensitivity of the assay. A combination of the CDR method with a commercial immunoreaction enhancing agent boosted the output signal and substantially reduced the antibody incubation time. The resulting ultra-high-speed western blot can be accomplished in 20 minutes without any loss in sensitivity. This method can be applied to western blots using both chemiluminescent and fluorescent detection. This simple protocol allows researchers to better explore the analysis of protein expression in many samples.
Conflict of interest statement
DISCLOSURES:
The authors have declared no competing interests directly relevant to the contents of this article.
Figures



References
-
- TOYOBO. IMMUNOREACTION ENHANCING: IE TECHNOLOGY. http://www.toyobousa.com/lifescience-immunoreaction-enhancing.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
