The mitotic protein NuMA plays a spindle-independent role in nuclear formation and mechanics
- PMID: 33044554
- PMCID: PMC7555356
- DOI: 10.1083/jcb.202004202
The mitotic protein NuMA plays a spindle-independent role in nuclear formation and mechanics
Abstract
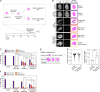
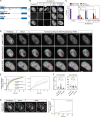
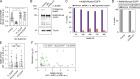
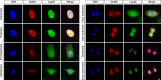
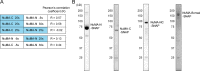
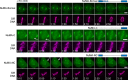
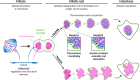
Eukaryotic cells typically form a single, round nucleus after mitosis, and failures to do so can compromise genomic integrity. How mammalian cells form such a nucleus remains incompletely understood. NuMA is a spindle protein whose disruption results in nuclear fragmentation. What role NuMA plays in nuclear integrity, and whether its perceived role stems from its spindle function, are unclear. Here, we use live imaging to demonstrate that NuMA plays a spindle-independent role in forming a single, round nucleus. NuMA keeps the decondensing chromosome mass compact at mitotic exit and promotes a mechanically robust nucleus. NuMA's C terminus binds DNA in vitro and chromosomes in interphase, while its coiled-coil acts as a central regulatory and structural element: it prevents NuMA from binding chromosomes at mitosis, regulates its nuclear mobility, and is essential for nuclear formation. Thus, NuMA plays a structural role over the cell cycle, building and maintaining the spindle and nucleus, two of the cell's largest structures.
© 2020 Serra-Marques et al.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

