Improvement of Functioning and Health With Ixekizumab in the Treatment of Active Nonradiographic Axial Spondyloarthritis in a 52-Week, Randomized, Controlled Trial
- PMID: 33044756
- PMCID: PMC9306696
- DOI: 10.1002/acr.24482
Improvement of Functioning and Health With Ixekizumab in the Treatment of Active Nonradiographic Axial Spondyloarthritis in a 52-Week, Randomized, Controlled Trial
Abstract
Objective: To evaluate the effect of ixekizumab on self-reported functioning and health in patients with active nonradiographic axial spondyloarthritis (SpA).
Methods: COAST-X was a randomized, controlled trial conducted in patients with nonradiographic axial SpA over 52 weeks. Participants were randomized at a ratio of 1:1:1 to receive 80 mg of ixekizumab subcutaneously every 4 weeks or 2 weeks or placebo for 52 weeks. Self-reported functioning and health end points included the Medical Outcomes Study Short Form 36 (SF-36) health survey, Assessment of Spondyloarthritis International Society (ASAS) health index, and European Quality of Life-5 Dimensions-5 Level (EQ-5D-5L) health-utility descriptive system.
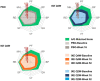
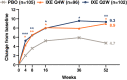
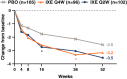
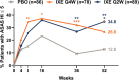
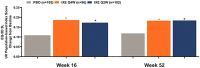
Results: Compared to placebo, ixekizumab treatment resulted in improvement of SF-36 physical component summary scores from baseline, with a score of 4.7 improving to 8.9 with ixekizumab therapy every 4 weeks (P < 0.05) and a score of 9.3 with ixekizumab therapy every 2 weeks (P < 0.01); the greatest improvements were observed in the domains of physical functioning, role-physical, and bodily pain at weeks 16 and 52. A higher proportion of patients receiving ixekizumab therapy every 2 weeks reported ≥3 improvements based on the ASAS health index from baseline to weeks 16 and 52 (P < 0.05). Significantly more patients receiving ixekizumab every 4 weeks reported improvements in "good health status" on the ASAS health index (ASAS score of ≤5) at weeks 16 and 52 (P < 0.05). Patients receiving ixekizumab reported improvements on the EQ-5D-5L compared to those who received placebo at week 16 (0.11 versus 0.17 for patients receiving treatment every 4 weeks and 0.19 for patients receiving treatment every 2 weeks; P < 0.05), which remained consistent at week 52. There were no clinical meaningful differences in responses based on the ixekizumab dosing regimen for patients who received ixekizumab therapy every 2 weeks or every 4 weeks.
Conclusion: In patients with nonradiographic axial SpA, therapy with ixekizumab was superior to placebo in the improvement of self-reported functioning and health at weeks 16 and 52.
Trial registration: ClinicalTrials.gov NCT02757352.
© 2020 The Authors. Arthritis Care & Research published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures
References
-
- Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017;390:73–84. - PubMed
-
- Rudwaleit M, van der Heijde D, Landewe R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. - PubMed
-
- Strand V, Rao SA, Shillington AC, Cifaldi MA, McGuire M, Ruderman EM. Prevalence of axial spondyloarthritis in United States rheumatology practices: Assessment of SpondyloArthritis International Society criteria versus rheumatology expert clinical diagnosis. Arthritis Care Res (Hoboken) 2013;65:1299–306. - PubMed
-
- Rudwaleit M, Sieper J. Referral strategies for early diagnosis of axial spondyloarthritis. Nat Rev Rheumatol 2012;8:262–8. - PubMed
-
- Protopopov M, Poddubnyy D. Radiographic progression in non‐radiographic axial spondyloarthritis. Expert Rev Clin Immunol 2018;14:525–33. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

