Chiral Induced Spin Selectivity Gives a New Twist on Spin-Control in Chemistry
- PMID: 33044813
- PMCID: PMC7676290
- DOI: 10.1021/acs.accounts.0c00485
Chiral Induced Spin Selectivity Gives a New Twist on Spin-Control in Chemistry
Abstract
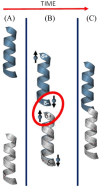
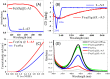
The electron's spin, its intrinsic angular momentum, is a quantum property that plays a critical role in determining the electronic structure of molecules. Despite its importance, it is not used often for controlling chemical processes, photochemistry excluded. The reason is that many organic molecules have a total spin zero, namely, all the electrons are paired. Even for molecules with high spin multiplicity, the spin orientation is usually only weakly coupled to the molecular frame of nuclei and hence to the molecular orientation. Therefore, controlling the spin orientation usually does not provide a handle on controlling the geometry of the molecular species during a reaction. About two decades ago, however, a new phenomenon was discovered that relates the electron's spin to the handedness of chiral molecules and is now known as the chiral induced spin selectivity (CISS) effect. It was established that the efficiency of electron transport through chiral molecules depends on the electron spin and that it changes with the enantiomeric form of a molecule and the direction of the electron's linear momentum. This property means that, for chiral molecules, the electron spin is strongly coupled to the molecular frame. Over the past few years, we and others have shown that this feature can be used to provide spin-control over chemical reactions and to perform enantioseparations with magnetic surfaces.In this Account, we describe the CISS effect and demonstrate spin polarization effects on chemical reactions. Explicitly, we describe a number of processes that can be controlled by the electron's spin, among them the interaction of chiral molecules with ferromagnetic surfaces, the multielectron oxidation of water, and enantiospecific electrochemistry. Interestingly, it has been shown that the effect also takes place in inorganic chiral oxides like copper oxide, aluminum oxide, and cobalt oxide. The CISS effect results from the coupling between the electron linear momentum and its spin in a chiral system. Understanding the implications of this interaction promises to reveal a previously unappreciated role for chirality in biology, where chiral molecules are ubiquitous, and opens a new avenue into spin-controlled processes in chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
The Importance of Spin State in Chiral Supramolecular Electronics.Front Chem. 2021 Aug 4;9:722727. doi: 10.3389/fchem.2021.722727. eCollection 2021. Front Chem. 2021. PMID: 34422770 Free PMC article. Review.
-
Effect of Chiral Molecules on the Electron's Spin Wavefunction at Interfaces.J Phys Chem Lett. 2020 Feb 20;11(4):1550-1557. doi: 10.1021/acs.jpclett.9b03487. Epub 2020 Feb 11. J Phys Chem Lett. 2020. PMID: 32013436 Free PMC article.
-
Chiral Induced Spin Selectivity and Its Implications for Biological Functions.Annu Rev Biophys. 2022 May 9;51:99-114. doi: 10.1146/annurev-biophys-083021-070400. Epub 2021 Dec 21. Annu Rev Biophys. 2022. PMID: 34932912 Review.
-
Optical Activity and Spin Polarization: The Surface Effect.J Am Chem Soc. 2023 Feb 22;145(7):3972-3977. doi: 10.1021/jacs.2c10456. Epub 2023 Feb 11. J Am Chem Soc. 2023. PMID: 36765468 Free PMC article.
-
Spintronics and chirality: spin selectivity in electron transport through chiral molecules.Annu Rev Phys Chem. 2015 Apr;66:263-81. doi: 10.1146/annurev-physchem-040214-121554. Epub 2015 Jan 19. Annu Rev Phys Chem. 2015. PMID: 25622190
Cited by
-
The effect of enantioselective chiral covalent organic frameworks and cysteine sacrificial donors on photocatalytic hydrogen evolution.Nat Commun. 2022 Oct 1;13(1):5768. doi: 10.1038/s41467-022-33501-8. Nat Commun. 2022. PMID: 36182957 Free PMC article.
-
Long-lived electronic spin qubits in single-walled carbon nanotubes.Nat Commun. 2023 Feb 15;14(1):848. doi: 10.1038/s41467-023-36031-z. Nat Commun. 2023. PMID: 36792597 Free PMC article.
-
Looking for chiral recognition in photoinduced bimolecular electron transfer using ultrafast spectroscopy.Phys Chem Chem Phys. 2023 Apr 26;25(16):11111-11120. doi: 10.1039/d3cp00760j. Phys Chem Chem Phys. 2023. PMID: 37017107 Free PMC article.
-
Assessing the Nature of Chiral-Induced Spin Selectivity by Magnetic Resonance.J Phys Chem Lett. 2021 Jul 15;12(27):6341-6347. doi: 10.1021/acs.jpclett.1c01447. Epub 2021 Jul 6. J Phys Chem Lett. 2021. PMID: 34228926 Free PMC article.
-
Chirality-Induced Spin Selectivity at the Molecular Level: A Different Perspective to Understand and Exploit the Phenomenon.J Phys Chem Lett. 2025 May 29;16(21):5358-5372. doi: 10.1021/acs.jpclett.5c00755. Epub 2025 May 21. J Phys Chem Lett. 2025. PMID: 40396560 Free PMC article. Review.
References
-
- Banerjee-Ghosh K.; Ben Dor O.; Tassinari F.; Capua E.; Yochelis S.; Capua A.; Yang S.-H.; Parkin S. S. P.; Sarkar S.; Kronik L.; Baczewski L. T.; Naaman R.; Paltiel Y. Separation of Enantiomers by Their Enantiospecific Interaction with Achiral Magnetic Substrates. Science 2018, 360, 1331–1334. 10.1126/science.aar4265. - DOI - PubMed
-
- Steiner U. E.; Ulrich T. Magnetic field effects in chemical kinetics and related phenomena. Chem. Rev. 1989, 89, 51–147. 10.1021/cr00091a003. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

