Intranasal mesenchymal stem cell therapy to boost myelination after encephalopathy of prematurity
- PMID: 33045105
- PMCID: PMC7821154
- DOI: 10.1002/glia.23919
Intranasal mesenchymal stem cell therapy to boost myelination after encephalopathy of prematurity
Abstract
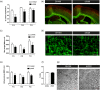
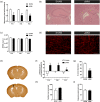
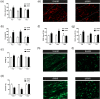
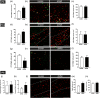
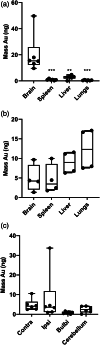
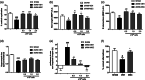
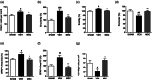
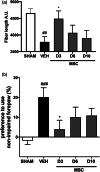
Encephalopathy of prematurity (EoP) is a common cause of long-term neurodevelopmental morbidity in extreme preterm infants. Diffuse white matter injury (dWMI) is currently the most commonly observed form of EoP. Impaired maturation of oligodendrocytes (OLs) is the main underlying pathophysiological mechanism. No therapies are currently available to combat dWMI. Intranasal application of mesenchymal stem cells (MSCs) is a promising therapeutic option to boost neuroregeneration after injury. Here, we developed a double-hit dWMI mouse model and investigated the therapeutic potential of intranasal MSC therapy. Postnatal systemic inflammation and hypoxia-ischemia led to transient deficits in cortical myelination and OL maturation, functional deficits and neuroinflammation. Intranasal MSCs migrated dispersedly into the injured brain and potently improved myelination and functional outcome, dampened cerebral inflammationand rescued OL maturation after dWMI. Cocultures of MSCs with primary microglia or OLs show that MSCs secrete factors that directly promote OL maturation and dampen neuroinflammation. We show that MSCs adapt their secretome after ex vivo exposure to dWMI milieu and identified several factors including IGF1, EGF, LIF, and IL11 that potently boost OL maturation. Additionally, we showed that MSC-treated dWMI brains express different levels of these beneficial secreted factors. In conclusion, the combination of postnatal systemic inflammation and hypoxia-ischemia leads to a pattern of developmental brain abnormalities that mimics the clinical situation. Intranasal delivery of MSCs, that secrete several beneficial factors in situ, is a promising strategy to restore myelination after dWMI and subsequently improve the neurodevelopmental outcome of extreme preterm infants in the future.
Keywords: diffuse white matter injury; encephalopathy of prematurity; mesenchymal stem cells; microglia; oligodendrocytes; preterm birth; regenerative medicine.
© 2020 The Authors. GLIA published by Wiley Periodicals LLC.
Figures

Similar articles
-
Modifying the Secretome of Mesenchymal Stem Cells Prolongs the Regenerative Treatment Window for Encephalopathy of Prematurity.Int J Mol Sci. 2024 Jun 12;25(12):6494. doi: 10.3390/ijms25126494. Int J Mol Sci. 2024. PMID: 38928201 Free PMC article.
-
Regenerative Therapies to Restore Interneuron Disturbances in Experimental Models of Encephalopathy of Prematurity.Int J Mol Sci. 2020 Dec 28;22(1):211. doi: 10.3390/ijms22010211. Int J Mol Sci. 2020. PMID: 33379239 Free PMC article.
-
The Potential of Stem Cell Therapy to Repair White Matter Injury in Preterm Infants: Lessons Learned From Experimental Models.Front Physiol. 2019 May 9;10:540. doi: 10.3389/fphys.2019.00540. eCollection 2019. Front Physiol. 2019. PMID: 31143126 Free PMC article. Review.
-
Timed fetal inflammation and postnatal hypoxia cause cortical white matter injury, interneuron imbalances, and behavioral deficits in a double-hit rat model of encephalopathy of prematurity.Brain Behav Immun Health. 2024 Jul 5;40:100817. doi: 10.1016/j.bbih.2024.100817. eCollection 2024 Oct. Brain Behav Immun Health. 2024. PMID: 39188404 Free PMC article.
-
The impact of trophic and immunomodulatory factors on oligodendrocyte maturation: Potential treatments for encephalopathy of prematurity.Glia. 2021 Jun;69(6):1311-1340. doi: 10.1002/glia.23939. Epub 2020 Nov 30. Glia. 2021. PMID: 33595855 Free PMC article. Review.
Cited by
-
Targeting Neuroinflammation in Preterm White Matter Injury: Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes.Cell Mol Neurobiol. 2025 Mar 12;45(1):23. doi: 10.1007/s10571-025-01540-6. Cell Mol Neurobiol. 2025. PMID: 40072734 Free PMC article. Review.
-
Development of a new treatment for preterm birth complications using amniotic fluid stem cell therapy.Histol Histopathol. 2023 Sep;38(9):965-974. doi: 10.14670/HH-18-607. Epub 2023 Mar 15. Histol Histopathol. 2023. PMID: 36971371 Review.
-
Outer radial glia promotes white matter regeneration after neonatal brain injury.Cell Rep Med. 2025 Mar 18;6(3):101986. doi: 10.1016/j.xcrm.2025.101986. Epub 2025 Feb 28. Cell Rep Med. 2025. PMID: 40023165 Free PMC article.
-
Hypoxic Preconditioning Enhances the Potential of Mesenchymal Stem Cells to Treat Neonatal Hypoxic-Ischemic Brain Injury.Stroke. 2025 Jul;56(7):1872-1882. doi: 10.1161/STROKEAHA.124.048964. Epub 2025 Apr 18. Stroke. 2025. PMID: 40248869 Free PMC article.
-
The Regulated Cell Death and Potential Interventions in Preterm Infants after Intracerebral Hemorrhage.Curr Neuropharmacol. 2023;21(7):1488-1503. doi: 10.2174/1570159X21666221117155209. Curr Neuropharmacol. 2023. PMID: 36397619 Free PMC article. Review.
References
-
- Baxi, E. G. , DeBruin, J. , Jin, J. , Strasburger, H. J. , Smith, M. D. , Orthmann‐Murphy, J. L. , … Calabresi, P. A. (2017). Lineage tracing reveals dynamic changes in oligodendrocyte precursor cells following cuprizone‐induced demyelination. Glia, 65(12), 2087–2098. 10.1002/glia.23229 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

