A Dinickel Catalyzed Cyclopropanation without the Formation of a Metal Carbene Intermediate
- PMID: 33045127
- PMCID: PMC8086810
- DOI: 10.1002/anie.202011602
A Dinickel Catalyzed Cyclopropanation without the Formation of a Metal Carbene Intermediate
Abstract
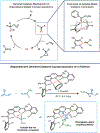
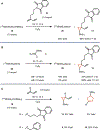
(NDI)Ni2 catalysts (NDI=naphthyridine-diimine) promote cyclopropanation reactions of 1,3-dienes using (Me3 Si)CHN2 . Mechanistic studies reveal that a metal carbene intermediate is not part of the catalytic cycle. The (NDI)Ni2 (CHSiMe3 ) complex was independently synthesized and found to be unreactive toward dienes. Based on DFT models, we propose an alternative mechanism that begins with a Ni2 -mediated coupling of (Me3 Si)CHN2 and the diene. N2 extrusion followed by radical C-C bond formation generates the cyclopropane product. This model reproduces the experimentally observed regioselectivity and diastereoselectivity of the reaction.
Keywords: carbenes; cyclopropane; homogeneous catalysis; metal-metal interactions; nickel.
© 2020 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures




References
-
- Li Y, Huang J-S, Zhou Z-Y, Che C-M, You X-Z, J. Am. Chem. Soc. 2002, 124, 13185–13193; - PubMed
- Waterman R, Hillhouse GL, J. Am. Chem. Soc. 2003, 125, 13350–13351; - PubMed
- Dai X, Warren TH, J. Am. Chem. Soc. 2004, 126, 10085–10094; - PubMed
- Kornecki KP, Briones JF, Boyarskikh V, Fullilove F, Autschbach J, Schrote KE, Lancaster KM, Davies HML, Berry JF, Science 2013, 342, 351–354; - PubMed
- Marquard SL, Bezpalko MW, Foxman BM, Thomas CM, J. Am. Chem. Soc. 2013, 135, 6018–6021; - PubMed
- Werlé C, Goddard R, Philipps P, Farès C, Früstner A, J. Am. Chem. Soc. 2016, 138, 3797–3805. - PubMed
-
- Rittle J, Green MT, Science 2010, 330, 933–937. - PubMed
-
- Collman JP, Chien AS, Eberspacher TA, Brauman JI, J. Am. Chem. Soc. 2000, 122, 11098–11100.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

