Neural cell adhesion molecule 1 is a novel autoantigen in membranous lupus nephritis
- PMID: 33045259
- PMCID: PMC8032825
- DOI: 10.1016/j.kint.2020.09.016
Neural cell adhesion molecule 1 is a novel autoantigen in membranous lupus nephritis
Abstract
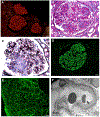
Membranous lupus nephritis is a frequent cause of nephrotic syndrome in patients with systemic lupus erythematosus. It has been shown in phospholipase A2 receptor positive membranous nephropathy that known antibodies can be detected within sera, determination of the target autoantigen can have diagnostic significance, inform prognosis, and enable non-invasive monitoring of disease activity. Here we utilized mass spectrometry for antigen discovery in laser captured microdissected glomeruli from formalin-fixed paraffin embedded tissue and tissue protein G immunoprecipitation studies to interrogate immune complexes from frozen kidney biopsy tissue. We identified neural cell adhesion molecule 1 (NCAM1) to be a target antigen in some cases of membranous lupus nephritis and within rare cases of primary membranous nephropathy. The prevalence of NCAM1 association was 6.6% of cases of membranous lupus nephritis and in 2.0% of primary membranous nephropathy cases. NCAM1 was found to colocalize with IgG within glomerular immune deposits by confocal microscopy. Additionally, serum from patients with NCAM1-associated membranous nephropathy showed reactivity to NCAM1 recombinant protein on Western blotting and by indirect immunofluorescence assay, demonstrating the presence of circulating antibodies. Thus, we propose that NCAM1 is a target autoantigen in a subset of patients with membranous lupus nephritis. Future studies are needed to determine whether anti-NCAM1 antibody levels correlate with disease activity or response to therapy.
Keywords: glomerular disease; immune complex; lupus nephritis; membranous nephropathy; serum testing; systemic lupus erythematosus.
Copyright © 2020 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Huong DL, Papo T, Beaufils H, et al. Renal involvement in systemic lupus erythematosus. A study of 180 patients from a single center. Medicine (Baltimore) 1999; 78: 148–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

