A whole blood test to measure SARS-CoV-2-specific response in COVID-19 patients
- PMID: 33045370
- PMCID: PMC7547312
- DOI: 10.1016/j.cmi.2020.09.051
A whole blood test to measure SARS-CoV-2-specific response in COVID-19 patients
Abstract
Objectives: To examine whether specific T-cell-responses to SARS-CoV-2 peptides can be detected in COVID-19 using a whole-blood experimental setting, which may be further explored as a potential diagnostic tool.
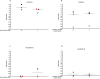
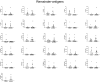
Methods: We evaluated interferon (IFN)-γ levels after stimulating whole-blood with spike and remainder-antigens peptides megapools (MP) derived from SARS-CoV-2 sequences; interleukin (IL)-1β, IL-1RA, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-17A, eotaxin, basic fibroblast growth factor (FGF), granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-γ, Interferon gamma-induced protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein (MIP)-1α, MIP-1β, Platelet-derived growth factor (PDGF), RANTES (regulated on activation, normal T cell expressed and secreted), tumour necrosis factor-alpha (TNF-α), vascular endothelial growth factor (VEGF) were also evaluated.
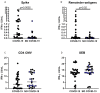
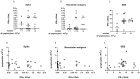
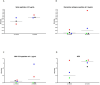
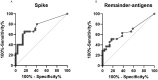
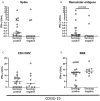
Results: IFN-γ-response to spike and remainder-antigens MPs was significantly increased in 35 COVID-19 patients compared with 29 'no COVID-19' individuals (medians spike-MP: 0.26 vs 0, p = 0.0002; medians remainder-antigens-MP: 0.07 vs 0.02; p = 0.02). This response was detected independently of patients' clinical parameters. IFN-γ-response to SARS-CoV-2-unrelated antigens cytomegalovirus (CMV) and Staphylococcal Enterotoxin B (SEB) was similar in COVID-19 compared with 'no COVID-19' individuals (median CMV: 3.46 vs 5.28, p = 0.16; median SEB: 12.68 vs 15.05; p = 0.1). In response to spike-MPs in COVID-19- compared with 'no COVID-19' -individuals, we found significant higher median of IL-2 (50.08 vs 0, p = 0.0018), IFN-γ (90.16 vs 0, p = 0.01), IL-4 (0.52 vs 0, p = 0.03), IL-13 (0.84 vs 0, p = 0.007) and MCP-1 (4602 vs 359.2, p = 0.05).
Conclusions: Immune response to SARS-CoV-2 peptides in a whole-blood assay is associated with COVID-19 and it is characterized by both Th1 and Th2 profile. This experimental approach may be useful for developing new T-cell based diagnostic tests for disease and vaccine settings.
Keywords: COVID-19; IFN-γ; Immune response; Multiplex analysis; SARS-CoV-2; Serology response; Specific response; T-cell based tests; Whole blood.
Copyright © 2020. Published by Elsevier Ltd.
Figures



References
-
- ECDC. Report on COVID-19 pandemic. 2020. https://www.ecdc.europa.eu/en/covid-19-pandemic
-
- Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323:2249–2251. - PubMed
-
- WHO . Vol. 2020. 2020. Clinical management of COVID-19. Interim guidance.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

