The vitamin D for COVID-19 (VIVID) trial: A pragmatic cluster-randomized design
- PMID: 33045402
- PMCID: PMC7547023
- DOI: 10.1016/j.cct.2020.106176
The vitamin D for COVID-19 (VIVID) trial: A pragmatic cluster-randomized design
Abstract
Objectives: To determine the effect of vitamin D supplementation on disease progression and post-exposure prophylaxis for COVID-19 infection. We hypothesize that high-dose vitamin D3 supplementation will reduce risk of hospitalization/death among those with recently diagnosed COVID-19 infection and will reduce risk of COVID-19 infection among their close household contacts.
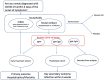
Methods: We report the rationale and design of a planned pragmatic, cluster randomized, double-blinded trial (N = 2700 in total nationwide), with 1500 newly diagnosed individuals with COVID-19 infection, together with up to one close household contact each (~1200 contacts), randomized to either vitamin D3 (loading dose, then 3200 IU/day) or placebo in a 1:1 ratio and a household cluster design. The study duration is 4 weeks. The primary outcome for newly diagnosed individuals is the occurrence of hospitalization and/or mortality. Key secondary outcomes include symptom severity scores among cases and changes in the infection (seroconversion) status for their close household contacts. Changes in vitamin D 25(OH)D levels will be assessed and their relation to study outcomes will be explored.
Conclusions: The proposed pragmatic trial will allow parallel testing of vitamin D3 supplementation for early treatment and post-exposure prophylaxis of COVID-19. The household cluster design provides a cost-efficient approach to testing an intervention for reducing rates of hospitalization and/or mortality in newly diagnosed cases and preventing infection among their close household contacts.
Keywords: COVID-19; Cluster randomization; Early treatment; Prophylaxis; SARS-CoV-2; Vitamin D.
Copyright © 2020. Published by Elsevier Inc.
References
-
- Greiller C.L., Suri R., Jolliffe D.A., Kebadze T., Hirsman A.G., Griffiths C.J., Johnston S.L., Martineau A.R. Vitamin D attenuates rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) and platelet-activating factor receptor (PAFR) in respiratory epithelial cells. J. Steroid Biochem. Mol. Biol. 2019;187:152–159. - PubMed
-
- Telcian A.G., Zdrenghea M.T., Edwards M.R., Laza-Stanca V., Mallia P., Johnston S.L., Stanciu L.A. Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antivir. Res. 2017;137:93–101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous