Early-life gut dysbiosis linked to juvenile mortality in ostriches
- PMID: 33046114
- PMCID: PMC7552511
- DOI: 10.1186/s40168-020-00925-7
Early-life gut dysbiosis linked to juvenile mortality in ostriches
Abstract
Background: Imbalances in the gut microbial community (dysbiosis) of vertebrates have been associated with several gastrointestinal and autoimmune diseases. However, it is unclear which taxa are associated with gut dysbiosis, and if particular gut regions or specific time periods during ontogeny are more susceptible. We also know very little of this process in non-model organisms, despite an increasing realization of the general importance of gut microbiota for health.
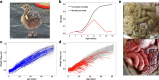
Methods: Here, we examine the changes that occur in the microbiome during dysbiosis in different parts of the gastrointestinal tract in a long-lived bird with high juvenile mortality, the ostrich (Struthio camelus). We evaluated the 16S rRNA gene composition of the ileum, cecum, and colon of 68 individuals that died of suspected enterocolitis during the first 3 months of life (diseased individuals), and of 50 healthy individuals that were euthanized as age-matched controls. We combined these data with longitudinal environmental and fecal sampling to identify potential sources of pathogenic bacteria and to unravel at which stage of development dysbiosis-associated bacteria emerge.
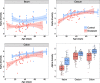
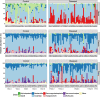
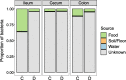
Results: Diseased individuals had drastically lower microbial alpha diversity and differed substantially in their microbial beta diversity from control individuals in all three regions of the gastrointestinal tract. The clear relationship between low diversity and disease was consistent across all ages in the ileum, but decreased with age in the cecum and colon. Several taxa were associated with mortality (Enterobacteriaceae, Peptostreptococcaceae, Porphyromonadaceae, Clostridium), while others were associated with health (Lachnospiraceae, Ruminococcaceae, Erysipelotrichaceae, Turicibacter, Roseburia). Environmental samples showed no evidence of dysbiosis-associated bacteria being present in either the food, water, or soil substrate. Instead, the repeated fecal sampling showed that pathobionts were already present shortly after hatching and proliferated in individuals with low microbial diversity, resulting in high mortality several weeks later.
Conclusions: Identifying the origins of pathobionts in neonates and the factors that subsequently influence the establishment of diverse gut microbiota may be key to understanding dysbiosis and host development. Video Abstract.
Keywords: Disease; Dysbacteriosis; Gastrointestinal tract; Gut microbiota; Inflammation; Microbial diversity.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Major shifts in gut microbiota during development and its relationship to growth in ostriches.Mol Ecol. 2019 May;28(10):2653-2667. doi: 10.1111/mec.15087. Epub 2019 May 11. Mol Ecol. 2019. PMID: 30916826
-
Measuring the gut microbiome in birds: Comparison of faecal and cloacal sampling.Mol Ecol Resour. 2018 May;18(3):424-434. doi: 10.1111/1755-0998.12744. Epub 2017 Dec 22. Mol Ecol Resour. 2018. PMID: 29205893
-
Intestinal Dysbiosis in Carriers of Carbapenem-Resistant Enterobacteriaceae.mSphere. 2020 Apr 29;5(2):e00173-20. doi: 10.1128/mSphere.00173-20. mSphere. 2020. PMID: 32350099 Free PMC article.
-
Low diversity gut microbiota dysbiosis: drivers, functional implications and recovery.Curr Opin Microbiol. 2018 Aug;44:34-40. doi: 10.1016/j.mib.2018.07.003. Epub 2018 Jul 20. Curr Opin Microbiol. 2018. PMID: 30036705 Free PMC article. Review.
-
Traumatic Spinal Cord Injury and the Gut Microbiota: Current Insights and Future Challenges.Front Immunol. 2020 May 8;11:704. doi: 10.3389/fimmu.2020.00704. eCollection 2020. Front Immunol. 2020. PMID: 32528463 Free PMC article. Review.
Cited by
-
Timing matters: age-dependent impacts of the social environment and host selection on the avian gut microbiota.Microbiome. 2022 Nov 26;10(1):202. doi: 10.1186/s40168-022-01401-0. Microbiome. 2022. PMID: 36434663 Free PMC article.
-
Influence of management practice on the microbiota of a critically endangered species: a longitudinal study of kākāpō chick faeces and associated nest litter.Anim Microbiome. 2022 Sep 30;4(1):55. doi: 10.1186/s42523-022-00204-w. Anim Microbiome. 2022. PMID: 36175950 Free PMC article.
-
Vitamin K2 supplementation improves impaired glycemic homeostasis and insulin sensitivity for type 2 diabetes through gut microbiome and fecal metabolites.BMC Med. 2023 May 5;21(1):174. doi: 10.1186/s12916-023-02880-0. BMC Med. 2023. PMID: 37147641 Free PMC article.
-
Composition and function of the Galapagos penguin gut microbiome vary with age, location, and a putative bacterial pathogen.Sci Rep. 2023 Apr 1;13(1):5358. doi: 10.1038/s41598-023-31826-y. Sci Rep. 2023. PMID: 37005428 Free PMC article.
-
Assessing the causes and consequences of gut mycobiome variation in a wild population of the Seychelles warbler.Microbiome. 2022 Dec 28;10(1):242. doi: 10.1186/s40168-022-01432-7. Microbiome. 2022. PMID: 36575553 Free PMC article.

