Plasma Membrane Fusion Is Specifically Impacted by the Molecular Structure of Membrane Sterols During Vegetative Development of Neurospora crassa
- PMID: 33046504
- PMCID: PMC7768248
- DOI: 10.1534/genetics.120.303623
Plasma Membrane Fusion Is Specifically Impacted by the Molecular Structure of Membrane Sterols During Vegetative Development of Neurospora crassa
Abstract
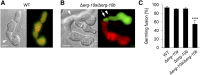
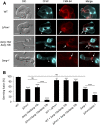
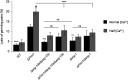
Cell-to-cell fusion is crucial for the development and propagation of most eukaryotic organisms. Despite this importance, the molecular mechanisms mediating this process are only poorly understood in biological systems. In particular, the step of plasma membrane merger and the contributing proteins and physicochemical factors remain mostly unknown. Earlier studies provided the first evidence of a role of membrane sterols in cell-to-cell fusion. By characterizing different ergosterol biosynthesis mutants of the fungus Neurospora crassa, which accumulate different ergosterol precursors, we show that the structure of the sterol ring system specifically affects plasma membrane merger during the fusion of vegetative spore germlings. Genetic analyses pinpoint this defect to an event prior to engagement of the fusion machinery. Strikingly, this effect is not observed during sexual fusion, suggesting that the specific sterol precursors do not generally block membrane merger, but rather impair subcellular processes exclusively mediating fusion of vegetative cells. At a colony-wide level, the altered structure of the sterol ring system affects a subset of differentiation processes, including vegetative sporulation and steps before and after fertilization during sexual propagation. Together, these observations corroborate the notion that the accumulation of particular sterol precursors has very specific effects on defined cellular processes rather than nonspecifically disturbing membrane functioning. Given the phenotypic similarities of the ergosterol biosynthesis mutants of N. crassa during vegetative fusion and of Saccharomyces cerevisiae cells undergoing mating, our data support the idea that yeast mating is evolutionarily and mechanistically more closely related to vegetative than sexual fusion of filamentous fungi.
Keywords: Neurospora crassa; cell fusion; ergosterol; mating; plasma membrane fusion.
Copyright © 2020 by the Genetics Society of America.
Figures





Similar articles
-
The Saccharomyces cerevisiae PRM1 homolog in Neurospora crassa is involved in vegetative and sexual cell fusion events but also has postfertilization functions.Genetics. 2009 Feb;181(2):497-510. doi: 10.1534/genetics.108.096149. Epub 2008 Dec 8. Genetics. 2009. PMID: 19064710 Free PMC article.
-
Accumulation of specific sterol precursors targets a MAP kinase cascade mediating cell-cell recognition and fusion.Proc Natl Acad Sci U S A. 2016 Oct 18;113(42):11877-11882. doi: 10.1073/pnas.1610527113. Epub 2016 Oct 5. Proc Natl Acad Sci U S A. 2016. PMID: 27708165 Free PMC article.
-
Identification and characterization of LFD1, a novel protein involved in membrane merger during cell fusion in Neurospora crassa.Mol Microbiol. 2014 Apr;92(1):164-82. doi: 10.1111/mmi.12545. Epub 2014 Mar 6. Mol Microbiol. 2014. PMID: 24673848
-
Cell fusion in the filamentous fungus, Neurospora crassa.Methods Mol Biol. 2008;475:21-38. doi: 10.1007/978-1-59745-250-2_2. Methods Mol Biol. 2008. PMID: 18979236 Review.
-
Cell fusion in Neurospora crassa.Curr Opin Microbiol. 2015 Dec;28:53-9. doi: 10.1016/j.mib.2015.08.002. Epub 2015 Sep 3. Curr Opin Microbiol. 2015. PMID: 26340439 Review.
Cited by
-
Analysis of the Putative Nucleoporin POM33 in the Filamentous Fungus Sordaria macrospora.J Fungi (Basel). 2021 Aug 24;7(9):682. doi: 10.3390/jof7090682. J Fungi (Basel). 2021. PMID: 34575720 Free PMC article.
References
-
- Athanasopoulos A., André B., Sophianopoulou V., and Gournas C., 2019. Fungal plasma membrane domains. FEMS Microbiol. Rev. 43: 642–673. doi: 10.1093/femsre/fuz022 - PubMed
-
- Bistis G. N., 1981. Chemotropic interactions between trichogynes and conidia of opposite mating-type in Neurospora crassa. Mycologia 73: 959–975. 10.1080/00275514.1981.12021425 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

