β-Adrenergic Receptors/Epac Signaling Increases the Size of the Readily Releasable Pool of Synaptic Vesicles Required for Parallel Fiber LTP
- PMID: 33046543
- PMCID: PMC7643300
- DOI: 10.1523/JNEUROSCI.0716-20.2020
β-Adrenergic Receptors/Epac Signaling Increases the Size of the Readily Releasable Pool of Synaptic Vesicles Required for Parallel Fiber LTP
Abstract
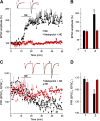
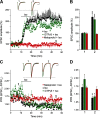
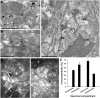
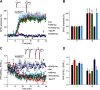
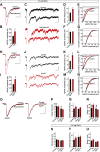
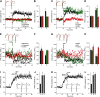
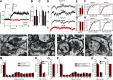
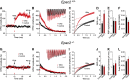
The second messenger cAMP is an important determinant of synaptic plasticity that is associated with enhanced neurotransmitter release. Long-term potentiation (LTP) at parallel fiber (PF)-Purkinje cell (PC) synapses depends on a Ca2+-induced increase in presynaptic cAMP that is mediated by Ca2+-sensitive adenylyl cyclases. However, the upstream signaling and the downstream targets of cAMP involved in these events remain poorly understood. It is unclear whether cAMP generated by β-adrenergic receptors (βARs) is required for PF-PC LTP, although noradrenergic varicosities are apposed in PF-PC contacts. Guanine nucleotide exchange proteins directly activated by cAMP [Epac proteins (Epac 1-2)] are alternative cAMP targets to protein kinase A (PKA) and Epac2 is abundant in the cerebellum. However, whether Epac proteins participate in PF-PC LTP is not known. Immunoelectron microscopy demonstrated that βARs are expressed in PF boutons. Moreover, activation of these receptors through their agonist isoproterenol potentiated synaptic transmission in cerebellar slices from mice of either sex, an effect that was insensitive to the PKA inhibitors (H-89, KT270) but that was blocked by the Epac inhibitor ESI 05. Interestingly, prior activation of these βARs occluded PF-PC LTP, while the β1AR antagonist metoprolol blocked PF-PC LTP, which was also absent in Epac2-/- mice. PF-PC LTP is associated with an increase in the size of the readily releasable pool (RRP) of synaptic vesicles, consistent with the isoproterenol-induced increase in vesicle docking in cerebellar slices. Thus, the βAR-mediated modulation of the release machinery and the subsequent increase in the size of the RRP contributes to PF-PC LTP.SIGNIFICANCE STATEMENT G-protein-coupled receptors modulate the release machinery, causing long-lasting changes in synaptic transmission that influence synaptic plasticity. Nevertheless, the mechanisms underlying synaptic responses to β-adrenergic receptor (βAR) activation remain poorly understood. An increase in the number of synaptic vesicles primed for exocytosis accounts for the potentiation of neurotransmitter release driven by βARs. This effect is not mediated by the canonical protein kinase A pathway but rather, through direct activation of the guanine nucleotide exchange protein Epac by cAMP. Interestingly, this βAR signaling via Epac is involved in long term potentiation at cerebellar granule cell-to-Purkinje cell synapses. Thus, the pharmacological activation of βARs modulates synaptic plasticity and opens therapeutic opportunities to control this phenomenon.
Keywords: Epac2 KO; RRP size; adrenergic receptors; neurotransmitter release; parallel fiber LTP; release machinery.
Copyright © 2020 the authors.
Figures
Similar articles
-
The activation of mGluR4 rescues parallel fiber synaptic transmission and LTP, motor learning and social behavior in a mouse model of Fragile X Syndrome.Mol Autism. 2023 Apr 7;14(1):14. doi: 10.1186/s13229-023-00547-4. Mol Autism. 2023. PMID: 37029391 Free PMC article.
-
β-Adrenergic receptors activate exchange protein directly activated by cAMP (Epac), translocate Munc13-1, and enhance the Rab3A-RIM1α interaction to potentiate glutamate release at cerebrocortical nerve terminals.J Biol Chem. 2013 Oct 25;288(43):31370-85. doi: 10.1074/jbc.M113.463877. Epub 2013 Sep 13. J Biol Chem. 2013. PMID: 24036110 Free PMC article.
-
Noradrenergic stabilization of heterosynaptic LTP requires activation of Epac in the hippocampus.Learn Mem. 2019 Jan 16;26(2):31-38. doi: 10.1101/lm.048660.118. Print 2019 Feb. Learn Mem. 2019. PMID: 30651375 Free PMC article.
-
Epac: A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell.Diabetes. 2004 Jan;53(1):5-13. doi: 10.2337/diabetes.53.1.5. Diabetes. 2004. PMID: 14693691 Free PMC article. Review.
-
Epac: defining a new mechanism for cAMP action.Annu Rev Pharmacol Toxicol. 2010;50:355-75. doi: 10.1146/annurev.pharmtox.010909.105714. Annu Rev Pharmacol Toxicol. 2010. PMID: 20055708 Review.
Cited by
-
The activation of mGluR4 rescues parallel fiber synaptic transmission and LTP, motor learning and social behavior in a mouse model of Fragile X Syndrome.Mol Autism. 2023 Apr 7;14(1):14. doi: 10.1186/s13229-023-00547-4. Mol Autism. 2023. PMID: 37029391 Free PMC article.
-
What Is the Role of Norepinephrine in Cerebellar Modulation and Stress-Induced Episodic Ataxia?Neurology. 2023 Feb 21;100(8):383-386. doi: 10.1212/WNL.0000000000206882. Neurology. 2023. PMID: 36806456 Free PMC article. No abstract available.
-
cAMP-EPAC-PKCε-RIM1α signaling regulates presynaptic long-term potentiation and motor learning.Elife. 2023 Apr 26;12:e80875. doi: 10.7554/eLife.80875. Elife. 2023. PMID: 37159499 Free PMC article.
-
The Expression of Epac2 and GluA3 in an Alzheimer's Disease Experimental Model and Postmortem Patient Samples.Biomedicines. 2023 Jul 25;11(8):2096. doi: 10.3390/biomedicines11082096. Biomedicines. 2023. PMID: 37626593 Free PMC article.
-
PXF-1 promotes synapse development at the neuromuscular junction in Caenorhabditis elegans.Front Mol Neurosci. 2022 Oct 13;15:945680. doi: 10.3389/fnmol.2022.945680. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36311020 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
