Web-Based Tailored Messaging to Increase Vaccination: A Randomized Clinical Trial
- PMID: 33046584
- PMCID: PMC7605085
- DOI: 10.1542/peds.2020-0669
Web-Based Tailored Messaging to Increase Vaccination: A Randomized Clinical Trial
Abstract
Background: To increase vaccine acceptance, we created a Web-based the "Vaccines and Your Baby" intervention (VAYB) that provided new parents with vaccine information messages tailored to vaccine beliefs and values. We evaluated the effectiveness of the VAYB by comparing timely uptake of infant vaccines to an untailored version of the intervention (UT) or usual care intervention (UC) only.
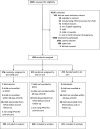
Methods: Between April 2016 and June 2019, we conducted a randomized clinical trial. Pregnant women and new parents were randomly assigned to the VAYB, UT, or UC arms. In the VAYB and UT arms, participants were exposed to interventions at 4 time points from pregnancy until their child was 15 months of age. The primary outcome was up-to-date status for recommended vaccines from birth to 200 days of age. A modified intent-to-treat analysis was conducted. Data were analyzed with logistic regression to generate odds ratios (ORs) and 95% confidence intervals (CIs).
Results: We enrolled 824 participants (276 VAYB, 274 UT, 274 UC), 143 (17.4%) of whom were lost to follow-up. The up-to-date rates in the VAYB, UT, and UC arms were 91.44%, 92.86%, and 92.31%, respectively. Infants in the VAYB arm were not more likely to be up to date than infants in the UC arm (OR = 0.89; 95% CI, 0.45-1.76) or in the UT arm (OR = 0.82; 95% CI, 0.42-1.63). The odds of being up to date did not differ between UT and UC arms (OR = 1.08; 95% CI, 0.54-2.18).
Conclusions: Delivering Web-based vaccine messages tailored to parents' vaccine attitudes and values did not positively impact the timely uptake of infant vaccines.
Trial registration: ClinicalTrials.gov NCT02665013.
Copyright © 2020 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
Figures
Comment in
-
The Toils, Trials, and Tribulations of Research on Childhood Vaccine Acceptance.Pediatrics. 2020 Nov;146(5):e2020013342. doi: 10.1542/peds.2020-013342. Epub 2020 Oct 12. Pediatrics. 2020. PMID: 33046587 No abstract available.
References
-
- Centers for Disease Control and Prevention Ten great public health achievements--United States, 2001–2010. MMWR Morbidity and Mortality Weekly Report. 2011;60(19):619–623 - PubMed
-
- Glanz JM, Newcomer SR, Narwaney KJ, et al. . A population-based cohort study of undervaccination in 8 managed care organizations across the United States. JAMA Pediatr. 2013;167(3):274–281 - PubMed
-
- Dempsey AF, Schaffer S, Singer D, Butchart A, Davis M, Freed GL. Alternative vaccination schedule preferences among parents of young children. Pediatrics. 2011;128(5):848–856 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous