Ultrathin-strut biodegradable polymer versus durable polymer drug-eluting stents: a meta-analysis
- PMID: 33046595
- PMCID: PMC7552849
- DOI: 10.1136/openhrt-2020-001394
Ultrathin-strut biodegradable polymer versus durable polymer drug-eluting stents: a meta-analysis
Abstract
Objectives: Determine whether an ultrathin biodegradable polymer sirolimus-eluting stent ('Orsiro'-BP-SES) has clinical benefits over second-generation durable polymer drug-eluting stents (DP-DES).
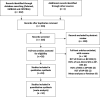
Methods: We conducted a prospective systematic review and meta-analysis of randomised clinical trials comparing Orsiro BP-SES against DP-DES (PROSPERO Registration: CRD42019147136). The primary outcome was target lesion failure (TLF): composite of cardiac death, target vessel myocardial infarction (TVMI) and clinically indicated target lesion revascularisation (TLR)) evaluated at the longest available follow-up.
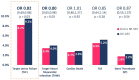
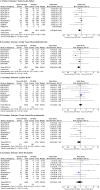
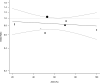
Results: Nine trials randomised 11 302 patients to either Orsiro BP-SES or DP-DES. At mean weighted follow-up of 2.8 years, the primary outcome (TLF) occurred in 501 of 6089 (8.2%) participants with BP-SES compared with 495 of 5213 (9.5%) participants with DP-DES. This equates to an absolute risk reduction of 1.3% in TLF in favour of Orsiro BP-SES (OR 0.82; 95% CI 0.69 to 0.98; p=0.03). This was driven by a reduction in TVMI (OR 0.80; 95% CI 0.65 to 0.98; p=0.03). There were no significant differences in other clinical endpoints: cardiac death, TLR and stent thrombosis.
Conclusion: The Orsiro BP-SES shows promising clinical outcomes in patients undergoing percutaneous coronary intervention compared with contemporary second-generation DES at a short to medium term follow-up. More research is warranted to evaluate performance over a longer follow-up period and in different clinical and lesion subsets.
Keywords: acute coronary syndrome; coronary artery disease; coronary intervention (PCI); coronary stenting; interventional cardiology.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
