Mexiletine in Myotonic Dystrophy Type 1: A Randomized, Double-Blind, Placebo-Controlled Trial
- PMID: 33046619
- PMCID: PMC7905778
- DOI: 10.1212/WNL.0000000000011002
Mexiletine in Myotonic Dystrophy Type 1: A Randomized, Double-Blind, Placebo-Controlled Trial
Erratum in
-
Mexiletine in Myotonic Dystrophy Type 1: A Randomized, Double-Blind, Placebo-Controlled Trial.Neurology. 2024 Jan 23;102(2):e207915. doi: 10.1212/WNL.0000000000207915. Epub 2023 Dec 14. Neurology. 2024. PMID: 38165356 Free PMC article. No abstract available.
Abstract
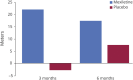
Objective: To assess mexiletine's long-term safety and effect on 6-minute walk distance in a well-defined cohort of patients with myotonic dystrophy type 1 (DM1).
Methods: We performed a randomized, double-blind, placebo-controlled trial of mexiletine (150 mg 3 times daily) to evaluate its efficacy and safety in a homogenous cohort of adult ambulatory patients with DM1. The primary outcome was change in 6-minute walk distance at 6 months. Secondary outcomes included changes in hand grip myotonia, strength, swallowing, forced vital capacity, lean muscle mass, Myotonic Dystrophy Health Index scores, and 24-hour Holter and ECG results at 3 and 6 months.
Results: Forty-two participants were randomized and 40 completed the 6-month follow-up (n = 20 in both groups). No significant effects of mexiletine were observed on 6-minute walk distance, but hand grip myotonia was improved with mexiletine treatment. There were no differences between the mexiletine and placebo groups with respect to the frequency or type of adverse events. Changes in PR, QRS, and QTc intervals were similar in mexiletine- and placebo-treated participants.
Conclusions: There was no benefit of mexiletine on 6-minute walk distance at 6 months. Although mexiletine had a sustained positive effect on objectively measured hand grip myotonia, this was not seen in measures reflecting participants' perceptions of their myotonia. No effects of mexiletine on cardiac conduction measures were seen over the 6-month follow-up period.
Classification of evidence: This study provides Class I evidence that for ambulatory patients with DM1, mexiletine does not significantly change 6-minute walk distance at 6 months.
© 2020 American Academy of Neurology.
Figures
References
-
- Brook JD, McCurrach ME, Harley HG, et al. . Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell 1992;68:799–808. - PubMed
-
- Fu YH, Pizzuti A, Fenwick RG, et al. . An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science 1992;255:1256–1258. - PubMed
-
- Mahadevan M, Tsilfidis C, Sabourin L, et al. . Myotonic dystrophy mutation: an unstable CTG repeat in the 3' untranslated region of the gene. Science 1992;255:1253–1255. - PubMed
-
- Talbot RG, Nimmo J, Julian DG, Clark RA, Neilson JM, Prescott LF. Treatment of ventricular arrhythmias with mexiletine (ko 1173). Lancet 1973;2:399–404. - PubMed
-
- Kwiecinski H, Ryniewicz B, Ostrzycki A. Treatment of myotonia with antiarrhythmic drugs. Acta Neurol Scand 1992;86:371–375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials