Clinical landscape of oncolytic virus research in 2020
- PMID: 33046622
- PMCID: PMC7552841
- DOI: 10.1136/jitc-2020-001486
Clinical landscape of oncolytic virus research in 2020
Abstract
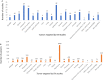
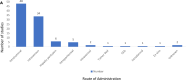
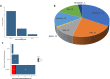
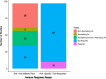
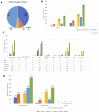
Oncolytic viruses (OVs) are a new class of cancer therapeutics. This review was undertaken to provide insight into the current landscape of OV clinical trials. A PubMed search identified 119 papers from 2000 to 2020 with 97 studies reporting data on 3233 patients. The viruses used, presence of genetic modifications and/or transgene expression, cancer types targeted, inclusion of combination strategies and safety profile were reported. In addition, information on viral bioshedding across the studies, including which tissues or body fluids were evaluated and how virus was detected (eg, PCR, plaque assay or both), is also reported. Finally, the number of studies evaluating antiviral and antitumor humoral and cellular immune responses were noted. We found that adenovirus (n=30) is the most common OV in clinical trials with approximately two-thirds (n=63) using modified or recombinant viral backbones and granulocyte-macrophage colony-stimulating factor (n=24) was the most common transgene. The most common tumors targeted were melanoma (n=1000) and gastrointestinal (GI; n=577) cancers with most using monotherapy OVs given by intratumoral (n=1482) or intravenous (n=1347) delivery. The most common combination included chemotherapy (n=36). Overall, OV treatment-related adverse events were low-grade constitutional and local injection site reactions. Viral shedding was frequently measured although many studies restricted this to blood and tumor tissue and used PCR only. While most studies did report antiviral antibody titers (n=63), only a minority of studies reported viral-specific T cell responses (n=10). Tumor immunity was reported in 48 studies and largely relied on general measures of immune activation (eg, tumor biopsy immunohistochemistry (n=25) and serum cytokine measurement (n=19)) with few evaluating tumor-specific immune responses (n=7). Objective responses were reported in 292 (9%) patients and disease control was achieved in 681 (21.1%) patients, although standard reporting criteria were only used in 53% of the trials. Completed clinical trials not reported in the peer-reviewed literature were not included in this review potentially underestimating the impact of OV treatment. These data provide insight into the current profile of OV clinical trials reporting and identifies potential gaps where further studies are needed to better define the role of OVs, alone and in combination, for patients with cancer.
Keywords: clinical trials as topic; immunotherapy; oncolytic virotherapy.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: HLK is an employee of Immuneering Corporation. DMM is a member of the scientific advisory board for Checkpoint Therapeutics, and has received honoraria from Pfizer, Merck Sharpe & Dome, Sanofi Genzyme and Regeneron. RH reports grant support from Bristol-Myers-Squibb and Novartis.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
