Filtering input fluctuations in intensity and in time underlies stochastic transcriptional pulses without feedback
- PMID: 33046652
- PMCID: PMC7604450
- DOI: 10.1073/pnas.2010849117
Filtering input fluctuations in intensity and in time underlies stochastic transcriptional pulses without feedback
Abstract
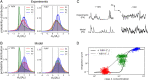
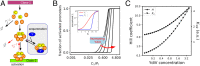
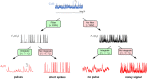
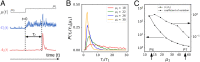
Stochastic pulsatile dynamics have been observed in an increasing number of biological circuits with known mechanism involving feedback control and bistability. Surprisingly, recent single-cell experiments in Escherichia coli flagellar synthesis showed that flagellar genes are activated in stochastic pulses without the means of feedback. However, the mechanism for pulse generation in these feedbackless circuits has remained unclear. Here, by developing a system-level stochastic model constrained by a large set of single-cell E. coli flagellar synthesis data from different strains and mutants, we identify the general underlying design principles for generating stochastic transcriptional pulses without feedback. Our study shows that an inhibitor (YdiV) of the transcription factor (FlhDC) creates a monotonic ultrasensitive switch that serves as a digital filter to eliminate small-amplitude FlhDC fluctuations. Furthermore, we find that the high-frequency (fast) fluctuations of FlhDC are filtered out by integration over a timescale longer than the timescale of the input fluctuations. Together, our results reveal a filter-and-integrate design for generating stochastic pulses without feedback. This filter-and-integrate mechanism enables a general strategy for cells to avoid premature activation of the expensive downstream gene expression by filtering input fluctuations in both intensity and time so that the system only responds to input signals that are both strong and persistent.
Keywords: filtering; gene regulation; stochastic modeling; transcriptional pulses; ultrasensitivity.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

