Cellular senescence-mediated exacerbation of Duchenne muscular dystrophy
- PMID: 33046751
- PMCID: PMC7550355
- DOI: 10.1038/s41598-020-73315-6
Cellular senescence-mediated exacerbation of Duchenne muscular dystrophy
Abstract
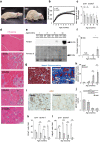
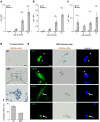
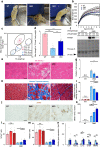
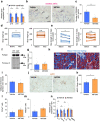
Duchenne muscular dystrophy (DMD) is a progressive disease characterised by chronic muscle degeneration and inflammation. Our previously established DMD model rats (DMD rats) have a more severe disease phenotype than the broadly used mouse model. We aimed to investigate the role of senescence in DMD using DMD rats and patients. Senescence was induced in satellite cells and mesenchymal progenitor cells, owing to the increased expression of CDKN2A, p16- and p19-encoding gene. Genetic ablation of p16 in DMD rats dramatically restored body weight and muscle strength. Histological analysis showed a reduction of fibrotic and adipose tissues invading skeletal muscle, with increased muscle regeneration. Senolytic drug ABT263 prevented loss of body weight and muscle strength, and increased muscle regeneration in rats even at 8 months-the late stage of DMD. Moreover, senescence markers were highly expressed in the skeletal muscle of DMD patients. In situ hybridization of CDKN2A confirmed the expression of it in satellite cells and mesenchymal progenitor cells in patients with DMD. Collectively, these data provide new insights into the integral role of senescence in DMD progression.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

