Myocarditis and inflammatory cardiomyopathy: current evidence and future directions
- PMID: 33046850
- PMCID: PMC7548534
- DOI: 10.1038/s41569-020-00435-x
Myocarditis and inflammatory cardiomyopathy: current evidence and future directions
Abstract
Inflammatory cardiomyopathy, characterized by inflammatory cell infiltration into the myocardium and a high risk of deteriorating cardiac function, has a heterogeneous aetiology. Inflammatory cardiomyopathy is predominantly mediated by viral infection, but can also be induced by bacterial, protozoal or fungal infections as well as a wide variety of toxic substances and drugs and systemic immune-mediated diseases. Despite extensive research, inflammatory cardiomyopathy complicated by left ventricular dysfunction, heart failure or arrhythmia is associated with a poor prognosis. At present, the reason why some patients recover without residual myocardial injury whereas others develop dilated cardiomyopathy is unclear. The relative roles of the pathogen, host genomics and environmental factors in disease progression and healing are still under discussion, including which viruses are active inducers and which are only bystanders. As a consequence, treatment strategies are not well established. In this Review, we summarize and evaluate the available evidence on the pathogenesis, diagnosis and treatment of myocarditis and inflammatory cardiomyopathy, with a special focus on virus-induced and virus-associated myocarditis. Furthermore, we identify knowledge gaps, appraise the available experimental models and propose future directions for the field. The current knowledge and open questions regarding the cardiovascular effects associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are also discussed. This Review is the result of scientific cooperation of members of the Heart Failure Association of the ESC, the Heart Failure Society of America and the Japanese Heart Failure Society.
Conflict of interest statement
C.T. is a consultant for Cardiotropic Labs, Miami, FL, USA. S.B.F. reports grants from Fresenius Medical Care and ENDI Foundation. J.M.H. holds equity in Heart Genomics. J.M.H. and B.H. are both inventors on a patent involving the use of RNA as a biomarker for myocarditis. The other authors declare no competing interests.
Figures


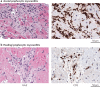

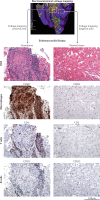


References
-
- Caforio AL, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

