Photocatalytic degradation of methylene blue under natural sunlight using iron titanate nanoparticles prepared by a modified sol-gel method
- PMID: 33047033
- PMCID: PMC7540765
- DOI: 10.1098/rsos.200708
Photocatalytic degradation of methylene blue under natural sunlight using iron titanate nanoparticles prepared by a modified sol-gel method
Abstract
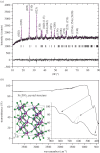
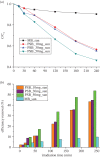
The aim of this work was to synthesize semiconducting oxide nanoparticles using a simple method with low production cost to be applied in natural sunlight for photocatalytic degradation of pollutants in waste water. Iron titanate (Fe2TiO5) nanoparticles with an orthorhombic structure were successfully synthesized using a modified sol-gel method and calcination at 750°C. The as-prepared Fe2TiO5 nanoparticles exhibited a moderate specific surface area. The mesoporous Fe2TiO5 nanoparticles possessed strong absorption in the visible-light region and the band gap was estimated to be around 2.16 eV. The photocatalytic activity was evaluated by the degradation of methylene blue under natural sunlight. The effect of parameters such as the amount of catalyst, initial concentration of the dye and pH of the dye solution on the removal efficiency of methylene blue was investigated. Fe2TiO5 showed high degradation efficiency in a strong alkaline medium that can be the result of the facilitated formation of OH radicals due to an increased concentration of hydroxyl ions.
Keywords: dye wastewater; iron titanate; methylene blue dye; photocatalytic degradation; sol–gel method.
© 2020 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures






References
-
- Aksu Z. 2005. Application of biosorption for the removal of organic pollutants: a review. Process Biochem. 40, 997–1026. (10.1016/j.procbio.2004.04.008) - DOI
-
- Brillas E, Martínez-Huitle CA. 2015. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: an updated review. Appl. Catal. B 166–167, 603–643. (10.1016/j.apcatb.2014.11.016) - DOI
-
- Chan S, Wu TY, Juan JC, Teh CY. 2011. Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. J. Chem. Technol. Biotechnol. 86, 1130–1158. (10.1002/jctb.2636) - DOI
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

