Is There Clinical Improvement Associated With Intradiscal Therapies? A Comparison Across Randomized Controlled Studies
- PMID: 33047622
- PMCID: PMC9344499
- DOI: 10.1177/2192568220963058
Is There Clinical Improvement Associated With Intradiscal Therapies? A Comparison Across Randomized Controlled Studies
Abstract
Study design: Post hoc comparison using single-site data from 4 multicenter randomized controlled trials.
Objectives: Discogenic back pain is associated with significant morbidity and medical cost. Several terminated, unreported randomized controlled trials have studied the effect of intradiscal biologic injections. Here we report single-center outcomes from these trials to determine if there is clinical improvement associated with these intradiscal injections.
Methods: Post hoc comparison was performed using single-site data from 4 similar multi-center randomized controlled trials. All trials evaluated an injectable therapy (growth factor, fibrin sealant, or stem cells) for symptomatic lumbar disc disease with near-identical inclusion and exclusion criteria. Demographics and patient reported outcomes were analyzed across treatment arms postinjection.
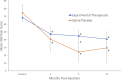
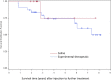
Results: A total of 38 patients were treated with biologic agents and 12 were treated with control saline injections. There was a significant decrease in visual analogue score (VAS) pain for both the investigational and saline groups up to 12 months postinjection (P < .01). There was no significant difference in VAS scores between the saline and investigational groups at 12 months. Similarly, there was significant improvement in patient-reported disability scores in both the investigational and saline groups at all time points. There were no significant differences in disability score improvement between the saline and investigational treatment groups at 12 months postinjection.
Conclusions: A single-center analysis of 4 randomized controlled studies demonstrated no difference in outcomes between therapeutic intradiscal agents (growth factor, fibrin sealant, or stem cells) and control saline groups. In all groups, patient reported pain and disability scores decreased significantly. Future studies are needed to evaluate the therapeutic benefit of any intradiscal injections.
Keywords: degenerative disc disease; discogenic back pain; intradiscal therapy; saline.
Conflict of interest statement
Figures




References
-
- Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. - PubMed
-
- Frank JW, Kerr MS, Brooker AS, et al. Disability resulting from occupational low back pain. Part I: What do we know about primary prevention? A review of the scientific evidence on prevention before disability begins. Spine (Phila Pa 1976). 1996;21:2908–2917. - PubMed
-
- Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N. The relative contributions of the disc and zygapophyseal joint in chronic low back pain. Spine (Phila Pa 1976). 1994;19:801–806. - PubMed
-
- de Schepper EI, Damen J, van Meurs JB, et al. The association between lumbar disc degeneration and low back pain: the influence of age, gender, and individual radiographic features. Spine (Phila Pa 1976). 2010;35:531–536. - PubMed
-
- An HS, Masuda K, Cs-Szabo G, et al. Biologic repair and regeneration of the intervertebral disk. J Am Acad Orthop Surg. 2011;19:450–452. - PubMed
LinkOut - more resources
Full Text Sources

