Renin-Angiotensin-Aldosterone System Inhibitors and COVID-19 Infection or Hospitalization: A Cohort Study
- PMID: 33048112
- PMCID: PMC7665332
- DOI: 10.1093/ajh/hpaa168
Renin-Angiotensin-Aldosterone System Inhibitors and COVID-19 Infection or Hospitalization: A Cohort Study
Abstract
Background: Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) may increase the risk of coronavirus disease 2019 (COVID-19) infection or affect disease severity. Prior studies have not examined risks by medication dose.
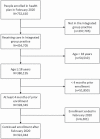
Methods: This retrospective cohort study included people aged ≥18 years enrolled in a US integrated healthcare system for at least 4 months as of 2/29/2020. Current ACEI and ARB use was identified from pharmacy data, and the estimated daily dose was calculated and standardized across medications. COVID-19 infections and hospitalizations were identified through 6/14/2020 from laboratory and hospitalization data. We used logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs), adjusting for race/ethnicity, obesity, and other covariates.
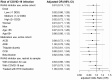
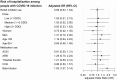
Results: Among 322,044 individuals, 826 developed COVID-19 infection. Among people using ACEI/ARBs, 204/56,105 developed COVID-19 (3.6 per 1,000 individuals) compared with 622/265,939 without ACEI/ARB use (2.3 per 1,000), yielding an adjusted OR of 0.91 (95% CI 0.74-1.12). For use of <1 defined daily dose (DDD) vs. nonuse, the adjusted OR for infection was 0.92 (95% CI 0.66-1.28); for 1 to <2 DDDs, 0.89 (95% CI 0.66-1.19); and for ≥2 DDDs, 0.92 (95% CI 0.72-1.18). The OR was similar for ACEIs and ARBs and in subgroups by age and sex. 26% of people with COVID-19 infection were hospitalized; the adjusted OR for hospitalization in relation to ACEI/ARB use was 0.98 (95% CI 0.63-1.54), and there was no association with dose.
Conclusions: These findings support current recommendations that individuals on these medications continue their use.
Keywords: COVID-19; angiotensin receptor blocker; angiotensin-converting enzyme inhibitor; blood pressure; coronavirus; hospitalization; hypertension; infection.
© American Journal of Hypertension, Ltd 2020. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

Update of
-
Renin-angiotensin-aldosterone system inhibitors and COVID-19 infection or hospitalization: a cohort study.medRxiv [Preprint]. 2020 Jul 7:2020.07.06.20120386. doi: 10.1101/2020.07.06.20120386. medRxiv. 2020. Update in: Am J Hypertens. 2021 Apr 20;34(4):339-347. doi: 10.1093/ajh/hpaa168. PMID: 32676610 Free PMC article. Updated. Preprint.
Comment in
-
ACE-2 Downregulation and Incidence of Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) Infection.Am J Hypertens. 2021 Apr 20;34(4):426. doi: 10.1093/ajh/hpaa191. Am J Hypertens. 2021. PMID: 33206164 Free PMC article. No abstract available.
References
-
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270–273. - PMC - PubMed
-
- American College of Cardiology. HFSA/ACC/AHA Statement Addresses Concerns RE: Using RAAS Antagonists in COVID-19. 2020. https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-.... Accessed October 23, 2020.
-
- Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005; 11:875–879. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

