Association of Cyclin-Dependent Kinases 4 and 6 Inhibitors With Survival in Patients With Hormone Receptor-Positive Metastatic Breast Cancer: A Systematic Review and Meta-analysis
- PMID: 33048129
- PMCID: PMC8094425
- DOI: 10.1001/jamanetworkopen.2020.20312
Association of Cyclin-Dependent Kinases 4 and 6 Inhibitors With Survival in Patients With Hormone Receptor-Positive Metastatic Breast Cancer: A Systematic Review and Meta-analysis
Erratum in
-
Errors in Abstract, Results, Discussion, Conclusions, and Table 1.JAMA Netw Open. 2020 Nov 2;3(11):e2030492. doi: 10.1001/jamanetworkopen.2020.30492. JAMA Netw Open. 2020. PMID: 33211103 Free PMC article. No abstract available.
Abstract
Importance: One of the most recent treatment regimens used for hormone receptor (HR)-positive, ERBB2 (formerly HER2)-negative metastatic breast cancer is treatment with the cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors and endocrine therapy (ET).
Objective: To assess overall survival (OS), progression-free survival (PFS), objective response rate, and adverse events, especially grades 3 and 4 adverse events, among patients with HR-positive, ERBB2-negative metastatic breast cancer who were treated with CDK4/6 inhibitors plus ET vs ET alone.
Data sources: A systematic search of PubMed, Embase, the main oncology conference of the European Society of Medical Oncology, and the American Society of Clinical Oncology and the San Antonio Breast Cancer Symposium databases for randomized clinical trials of CDK4/6 inhibitors plus ET vs ET for HR-positive, ERBB2-negative metastatic breast cancer. Searches were performed up to March 30, 2020.
Study selection: A total of 472 records were assessed in PubMed and Embase by 2 authors, including studies, international meeting reports, and reviews. Inclusion criteria were English-language phase 2 or 3 randomized clinical trials of HR-positive, ERBB2-negative metastatic breast cancer, with patients randomly assigned to receive CDK4/6 inhibitors plus ET or ET alone, and having OS or PFS outcomes. The exclusion criteria were phase 1 trials, retrospective studies, or studies without survival outcomes. Excluding the references, 16 articles were relevant. After excluding studies based on exclusion criteria, 9 studies were considered eligible for this meta-analysis.
Data extraction and synthesis: Two researchers independently extracted data and assessed potential bias. Data assessment followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses reporting guideline. The results were pooled using a fixed-effect model.
Main outcomes and measures: Study heterogeneity was assessed using the I2 statistic. Hazard ratios (HRs) and 95% CIs were used to evaluate PFS, OS, and subgroup analyses. Overall response and 95% CIs were used to evaluate the objective response rate and grade 3 or 4 adverse events. The primary outcome was OS.
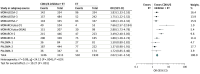
Results: In total, 9 studies that included a total of 5043 patients with metastatic breast cancer were assessed in this meta-analysis. Overall, the addition of CDK4/6 inhibitors to ET was associated with a statistically significant benefit to OS (HR, 1.33; 95% CI, 1.19-1.48; P < .001). Compared with ET alone, treatment with CDK4/6 inhibitors plus ET was associated with improved OS for the following subgroups: first-line therapy (HR, 1.35; 95% CI, 1.18-1.54; P < .001), second-line therapy (HR, 1.30; 95% CI, 1.09-1.54; P < .001), premenopausal women (HR, 1.32; 95% CI, 1.04-1.66; P < .001), postmenopausal women (HR, 1.34; 95% CI, 1.18-1.52; P < .001), visceral metastasis (HR, 1.31; 95% CI, 1.12-1.53; P < .001), bone-only metastasis (HR, 1.22; 95% CI, 0.88-1.68; P < .001), age younger than 65 years (HR, 1.25; 95% CI, 1.06-1.49; P < .001), and age 65 years or older (HR, 1.38; 95% CI, 1.11-1.72; P < .001). The addition of CDK4/6 inhibitors to ET was also associated with significant PFS benefit (HR, 1.84; 95% CI, 1.70-1.98; P < .001) and objective response rate benefit (odds ratio, 2.02; 95% CI, 1.61-2.53; P < .001). However, the use of CDK4/6 inhibitors in combination with ET was associated with significantly increased risk of grade 3 or 4 adverse events compared with ET alone, including neutropenia (HR, 57.05; 95% CI, 38.26-85.05; P < .001), leukopenia (HR, 36.36; 95% CI, 19.35-68.34; P < .001), and diarrhea (HR, 4.97; 95% CI, 2.84-8.69; P < .001).
Conclusions and relevance: This meta-analysis indicated that, compared with ET alone, treatment with CDK4/6 inhibitors plus ET was associated with significantly improved OS, PFS, and objective response rate among patients with HR-positive, ERBB2-negative metastatic breast cancer.
Conflict of interest statement
Figures


Comment in
-
In Support of CDK4/6 Inhibitors-A Meta-analysis of Available Randomized Data.JAMA Netw Open. 2020 Oct 1;3(10):e2021062. doi: 10.1001/jamanetworkopen.2020.21062. JAMA Netw Open. 2020. PMID: 33048125 No abstract available.
References
-
- Ayyagari R, Tang D, Patterson-Lomba O, et al. Progression-free survival with endocrine-based therapies following progression on non-steroidal aromatase inhibitor among postmenopausal women with hormone receptor positive, human epidermal growth factor receptor-2 negative metastatic breast cancer: a network meta-analysis. Curr Med Res Opin. 2018;34(9):1645-1652. doi: 10.1080/03007995.2018.1479246 - DOI - PubMed
-
- Lasheen S, Shohdy KS, Kassem L, Abdel-Rahman O. Fatigue, alopecia and stomatitis among patients with breast cancer receiving cyclin-dependent kinase 4 and 6 inhibitors: a systematic review and meta-analysis. Expert Rev Anticancer Ther. 2017;17(9):851-856. doi: 10.1080/14737140.2017.1355242 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

