Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): a randomized, controlled trial
- PMID: 33048195
- PMCID: PMC7550843
- DOI: 10.1007/s00134-020-06266-1
Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): a randomized, controlled trial
Abstract
Purpose: Contemporary trauma resuscitation prioritizes control of bleeding and uses major haemorrhage protocols (MHPs) to prevent and treat coagulopathy. We aimed to determine whether augmenting MHPs with Viscoelastic Haemostatic Assays (VHA) would improve outcomes compared to Conventional Coagulation Tests (CCTs).
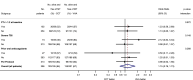
Methods: This was a multi-centre, randomized controlled trial comparing outcomes in trauma patients who received empiric MHPs, augmented by either VHA or CCT-guided interventions. Primary outcome was the proportion of subjects who, at 24 h after injury, were alive and free of massive transfusion (10 or more red cell transfusions). Secondary outcomes included 28-day mortality. Pre-specified subgroups included patients with severe traumatic brain injury (TBI).
Results: Of 396 patients in the intention to treat analysis, 201 were allocated to VHA and 195 to CCT-guided therapy. At 24 h, there was no difference in the proportion of patients who were alive and free of massive transfusion (VHA: 67%, CCT: 64%, OR 1.15, 95% CI 0.76-1.73). 28-day mortality was not different overall (VHA: 25%, CCT: 28%, OR 0.84, 95% CI 0.54-1.31), nor were there differences in other secondary outcomes or serious adverse events. In pre-specified subgroups, there were no differences in primary outcomes. In the pre-specified subgroup of 74 patients with TBI, 64% were alive and free of massive transfusion at 24 h compared to 46% in the CCT arm (OR 2.12, 95% CI 0.84-5.34).
Conclusion: There was no difference in overall outcomes between VHA- and CCT-augmented-major haemorrhage protocols.
Keywords: Coagulopathy; Haemorrhage; Thrombelastography; Thromboelastometry; Trauma.
Conflict of interest statement
K.Baksaas-Aasen, L.S.Gall, J.Stensballe, N.P.Juffermans, N.Curry, C.Rourke, S.Gillespie, J.Murphy, R.Maroni, P.Vulliamy, H.H.Henriksen, K.Holst Pedersen, K.M. Kolstadbraaten, M.R.Wirtz, D.J.B. Kleinveld, N.Schäfer, S.Chinna, P.A.Naess, J.C.Goslings, S.Eaglestone and S.Stanworth declare no conflicts of interest. M.Maegele has received honoraria for lectures and speakers’ bureaus, congress travel support as well as financial support for research projects from Astra Zeneca, Bayer, CSL Behring, IL-Werfen/TEM International, LFB Biomedicaments and Portola Inc. A. Brooks has received a research grant from Haemonetics Corp. in the form of cartridges and reagent support. He has also served on advisory panel for Haemonetics Corp. and TEM International and received honoraria for education lectures for Johnson and Johnson. P.I. Johansson´s has received unrestricted research grants from Haemonetics Corp. and Octapharma AG. C.Gaarder has received honoraria for lectures from Octapharma and research grant support from Haemonetics and TEM International in the form of device and reagent support. She has also previously served on the advisory board for Nycomed. K.Brohi and R.Davenport have received research grant support from TEM International in the form of device and reagent support. K.Brohi has previously served on external advisory panels for Haemonetics Corp, TEM International, CSL Behring, Bayer and Astra Zeneca.
Figures


Comment in
-
Clinical validation of precision medicine protocols: the last mile is the longest.Intensive Care Med. 2021 Jan;47(1):80-82. doi: 10.1007/s00134-020-06301-1. Epub 2020 Oct 29. Intensive Care Med. 2021. PMID: 33123786 No abstract available.
-
Trauma patients do not benefit from a viscoelastic haemostatic assay-guided protocol, but why?Intensive Care Med. 2021 Jun;47(6):726-727. doi: 10.1007/s00134-021-06396-0. Epub 2021 Apr 15. Intensive Care Med. 2021. PMID: 33860340 No abstract available.
-
Trauma-induced coagulopathy.Intensive Care Med. 2022 Nov;48(11):1642-1645. doi: 10.1007/s00134-022-06834-7. Epub 2022 Aug 4. Intensive Care Med. 2022. PMID: 35925321 No abstract available.
References
-
- Cole E, Weaver A, Gall L et al (2019) A decade of damage control resuscitation: new transfusion practice, new survivors, new directions. Ann Surg. 10.1097/SLA.0000000000003657 - PubMed
-
- NICE recommendations Major Trauma (NG39) published February 2016. https://www.nice.org.uk/guidance/ng39. Accessed 1st of February 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

