The long-acting C5 inhibitor, ravulizumab, is efficacious and safe in pediatric patients with atypical hemolytic uremic syndrome previously treated with eculizumab
- PMID: 33048203
- PMCID: PMC7910247
- DOI: 10.1007/s00467-020-04774-2
The long-acting C5 inhibitor, ravulizumab, is efficacious and safe in pediatric patients with atypical hemolytic uremic syndrome previously treated with eculizumab
Erratum in
-
Correction to: The long-acting C5 inhibitor, ravulizumab, is efficacious and safe in pediatric patients with atypical hemolytic uremic syndrome previously treated with eculizumab.Pediatr Nephrol. 2021 Apr;36(4):1033. doi: 10.1007/s00467-020-04874-z. Pediatr Nephrol. 2021. PMID: 33296010 Free PMC article. No abstract available.
Abstract
Background: Atypical hemolytic uremic syndrome (aHUS) is a rare, complement-mediated disease associated with poor outcomes if untreated. Ravulizumab, a long-acting C5 inhibitor developed through minimal, targeted modifications to eculizumab was recently approved for the treatment of aHUS. Here, we report outcomes from a pediatric patient cohort from the ravulizumab clinical trial (NCT03131219) who were switched from chronic eculizumab to ravulizumab treatment.
Methods: Ten patients received a loading dose of ravulizumab on Day 1, followed by maintenance doses administered initially on Day 15, and then, every 4-8 weeks thereafter, depending on body weight. All patients completed the initial evaluation period of 26 weeks and entered the extension period.
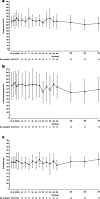
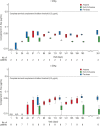
Results: No patients required dialysis at any point throughout the study. The median estimated glomerular filtration rate values remained stable during the trial: 99.8 mL/min/1.73m2 at baseline, 93.5 mL/min/1.73m2 at 26 weeks, and 104 mL/min/1.73m2 at 52 weeks. At last available follow-up, all patients were in the same chronic kidney disease stage as recorded at baseline. Hematologic variables (platelets, lactate dehydrogenase, and hemoglobin) also remained stable throughout the initial evaluation period and up to the last available follow-up. All patients experienced adverse events; the most common were upper respiratory tract infection (40%) and oropharyngeal pain (30%). There were no meningococcal infections reported, no deaths occurred, and no patients discontinued during the study.
Conclusions: Overall, treatment with ravulizumab in pediatric patients with aHUS who were previously treated with eculizumab resulted in stable kidney and hematologic parameters, with no unexpected safety concerns when administered every 4-8 weeks.
Trial registration: Trial identifiers: Trial ID: ALXN1210-aHUS-312 Clinical trials.gov : NCT03131219 EudraCT number: 2016-002499-29 Graphical abstract.
Keywords: Atypical hemolytic uremic syndrome; Children; Complement; Eculizumab; Hemolytic uremic syndrome; Ravulizumab; Thrombotic microangiopathy.
Conflict of interest statement
Kazuki Tanaka has received research support and consultation fees from Alexion Pharma GK. Brigitte Adams has no competing interests to declare. Alvaro Madrid Aris has no competing interests to declare. Naoya Fujita has no competing interests to declare. Masayo Ogawa is an employee and shareholder of Alexion Pharmaceuticals Inc. Stephan Ortiz is an employee and shareholder of Alexion Pharmaceuticals Inc. Marc Vallee is a shareholder of Alexion Pharmaceuticals Inc. Larry A. Greenbaum has received research support and consultation fees from Alexion Pharmaceuticals. He also serves on the scientific advisory board of the International aHUS Registry, sponsored by Alexion Pharmaceuticals.
Figures



References
-
- Campistol JM, Arias M, Ariceta G, Blasco M, Espinosa L, Espinosa M, Grinyo JM, Macia M, Mendizabal S, Praga M, Roman E, Torra R, Valdes F, Vilalta R, Rodriguez de Cordoba S. An update for atypical haemolytic uraemic syndrome: diagnosis and treatment. A consensus document. Nefrologia. 2015;35:421–447. doi: 10.1016/j.nefro.2015.07.005. - DOI - PubMed
-
- Fremeaux-Bacchi V, Fakhouri F, Garnier A, Bienaime F, Dragon-Durey MA, Ngo S, Moulin B, Servais A, Provot F, Rostaing L, Burtey S, Niaudet P, Deschenes G, Lebranchu Y, Zuber J, Loirat C. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8:554–562. doi: 10.2215/CJN.04760512. - DOI - PMC - PubMed
-
- Noris M, Caprioli J, Bresin E, Mossali C, Pianetti G, Gamba S, Daina E, Fenili C, Castelletti F, Sorosina A, Piras R, Donadelli R, Maranta R, van der Meer I, Conway EM, Zipfel PF, Goodship TH, Remuzzi G. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5:1844–1859. doi: 10.2215/CJN.02210310. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

