Evolving Landscape of New Drug Approval in Japan and Lags from International Birth Dates: Retrospective Regulatory Analysis
- PMID: 33048367
- PMCID: PMC8246743
- DOI: 10.1002/cpt.2080
Evolving Landscape of New Drug Approval in Japan and Lags from International Birth Dates: Retrospective Regulatory Analysis
Abstract
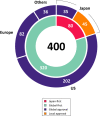
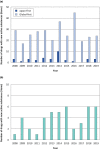
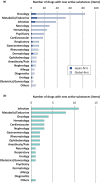
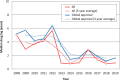
The Pharmaceuticals and Medical Devices Agency (PMDA) has approved hundreds of new drugs in recent years. We retrospectively analyzed the new drugs approved in Japan from 2008 to 2019, and identify the first-in-world approvals and clarify the current drug lag. The new drug and the drug lag were defined as a drug with a new active substance and a difference between the approval date in Japan and the international birth date, respectively. Among 400 new drugs approved in Japan during the last 12 years, 80 (20.0%) were first approved in Japan, and 320 were outside Japan (the United States: 202, 50.5%; Europe: 82, 20.5%; other regions: 36, 9.0%). Of these, 45 new drugs have not yet been approved outside Japan, and the remaining 355 have been globally approved in Japan and overseas. The number of new drug approvals were the largest in oncology followed by metabolic/endocrine and infectious diseases. The median drug lags (year) among all 400 new drugs and 355 new drugs with global approvals were 4.3 and 4.7 in the first tertile (2008-2011), 1.5 and 2.6 in the second tertile (2012-2015), and reduced to 1.3 and 2.2 in the third tertile (2016-2019), respectively. Substantial drug lag remains in neurology, psychiatry, and therapeutic areas where the number of new drug approvals was relatively small. Collectively, one-fifth of the new drugs approved in Japan are first-in-world approvals. Drug lag has been greatly decreased, although it still exists.
© 2020 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures
References
-
- Hirai, Y. , Kinoshita, H. , Kusama, M. , Yasuda, K. , Sugiyama, Y. & Ono, S. Delays in new drug applications in Japan and industrial R&D strategies. Clin. Pharmacol. Ther. 87, 212–218 (2010). - PubMed
-
- Tsuji, K. & Tsutani, K. Approval of new drugs 1999–2007: comparison of the US, the EU and Japan situations. J. Clin. Pharm. Ther. 35, 289–301 (2010). - PubMed
-
- Morgan, J.P. The drug lag: good or bad? J. Am. Pharm. Assoc. 17, 553–555 (1977). - PubMed
-
- Yamada, T. , Kusama, M. , Hirai, Y. , Arnold, F. , Sugiyama, Y. & Ono, S. Analysis of pharmaceutical safety‐related regulatory actions in Japan: do tradeoffs exist between safer drugs and launch delay? Ann. Pharmacother. 44, 1976–1985 (2010). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

