Thyroid hormones in diabetes, cancer, and aging
- PMID: 33048427
- PMCID: PMC7681062
- DOI: 10.1111/acel.13260
Thyroid hormones in diabetes, cancer, and aging
Abstract
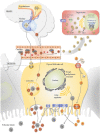
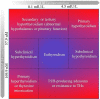
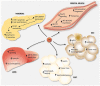
Thyroid function is central in the control of physiological and pathophysiological processes. Studies in animal models and human research have determined that thyroid hormones modulate cellular processes relevant for aging and for the majority of age-related diseases. While several studies have associated mild reductions on thyroid hormone function with exceptional longevity in animals and humans, alterations in thyroid hormones are serious medical conditions associated with unhealthy aging and premature death. Moreover, both hyperthyroidism and hypothyroidism have been associated with the development of certain types of diabetes and cancers, indicating a great complexity of the molecular mechanisms controlled by thyroid hormones. In this review, we describe the latest findings in thyroid hormone research in the field of aging, diabetes, and cancer, with a special focus on hepatocellular carcinomas. While aging studies indicate that the direct modulation of thyroid hormones is not a viable strategy to promote healthy aging or longevity and the development of thyromimetics is challenging due to inefficacy and potential toxicity, we argue that interventions based on the use of modulators of thyroid hormone function might provide therapeutic benefit in certain types of diabetes and cancers.
Keywords: cancer; diabetes; health span; hyperthyroidism; hypothyroidism; life span; thyroid hormones.
© 2020 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Adami, H.‐O. , Chow, W.‐H. , Nyren, O. , Berne, C. , Linet, M. S. , Ekbom, A. , Wolk, A. , McLaughlin, J. K. , & Fraumeni, J. F. (1996). Excess risk of primary liver cancer in patients with diabetes mellitus. Journal of the National Cancer Institute, 88, 1472–1477. 10.1093/jnci/88.20.1472 - DOI - PubMed
-
- Aguayo‐Mazzucato, C. , Zavacki, A. M. , Marinelarena, A. , Hollister‐Lock, J. , El Khattabi, I. , Marsili, A. , Weir, G. C. , Sharma, A. , Larsen, P. R. , & Bonner‐Weir, S. (2013). Thyroid hormone promotes postnatal rat pancreatic beta‐cell development and glucose‐responsive insulin secretion through MAFA. Diabetes, 62, 1569–1580. 10.2337/db12-0849 - DOI - PMC - PubMed
-
- Aguayo‐Mazzucato, C. , Lee Jr, T. B. , Matzko, M. , Dilenno, A. , Rezanejad, H. , Ramadoss, P. , Scanlan, T. , Zavacki, A. M. , Larsen, P. R. , Hollenberg, A. , Colton, C. , Sharma, A. , & Bonner‐Weir, S. (2018). T3 induces both markers of maturation and aging in pancreatic beta‐cells. Diabetes, 67, 1322–1331. 10.2337/db18-0030 - DOI - PMC - PubMed
-
- Aguayo‐Mazzucato, C. , DiIenno, A. , Hollister‐Lock, J. , Cahill, C. , Sharma, A. , Weir, G. , Colton, C. , & Bonner‐Weir, S. (2015). MAFA and T3 drive maturation of both fetal human islets and insulin‐producing cells differentiated from hESC. The Journal of Clinical Endocrinology & Metabolism, 100(10), 3651–3659. 10.1210/jc.2015-2632 - DOI - PMC - PubMed

