Increased marrow adipogenesis does not contribute to age-dependent appendicular bone loss in female mice
- PMID: 33048436
- PMCID: PMC7681065
- DOI: 10.1111/acel.13247
Increased marrow adipogenesis does not contribute to age-dependent appendicular bone loss in female mice
Abstract
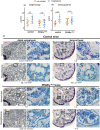
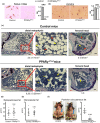
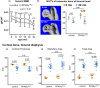
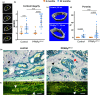
Marrow adipocytes and osteoblasts differentiate from common mesenchymal progenitors in a mutually exclusive manner, and diversion of these progenitors toward adipocytes in old age has been proposed to account for the decline in osteoblasts and the development of involutional osteoporosis. This idea has been supported by evidence that thiazolidinedione (TZD)-induced activation of PPARγ, the transcription factor required for adipocyte differentiation, increases marrow fat and causes bone loss. We functionally tested this hypothesis using C57BL/6J mice with conditional deletion of PPARγ from early mesenchymal progenitors targeted by the Prx1-Cre transgene. Using a longitudinal littermate-controlled study design, we observed that PPARγ is indispensable for TZD-induced increase in marrow adipocytes in 6-month-old male mice, and age-associated increase in marrow adipocytes in 22-month-old female mice. In contrast, PPARγ is dispensable for the loss of cortical and trabecular bone caused by TZD or old age. Instead, PPARγ restrains age-dependent development of cortical porosity. These findings do not support the long-standing hypothesis that increased marrow adipocyte differentiation contributes to bone loss in old age but reveal a novel role of mesenchymal cell PPARγ in the maintenance of cortical integrity.
Keywords: PPARγ; aging; osteoblasts; osteoporosis; porosity; rosiglitazone.
© 2020 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
D.Z. is co‐founder and advisor to Unity Biotechnology, which develops small‐molecule senolytic drugs for age‐related disease. The other authors have no conflicts.
Figures



References
-
- Akune, T. , Ohba, S. , Kamekura, S. , Yamaguchi, M. , Chung, U. , Kubota, N. , Terauchi, Y. , Harada, Y. , Azuma, Y. , Nakamura, K. , Kadowaki, T. , & Kawaguchi, H. (2004). PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. Journal of Clinical Investigation, 113(6), 846–855. - PMC - PubMed
-
- Ali, A. A. , Weinstein, R. S. , Stewart, S. A. , Parfitt, A. M. , Manolagas, S. C. , & Jilka, R. L. (2005). Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology, 146(3), 1226–1235. - PubMed
-
- Almeida, M. , Iyer, S. , Martin‐Millan, M. , Bartell, S. M. , Han, L. , Ambrogini, E ., Onal, M. , Xiong, J. , Weinstein, R. S. , Jilka, R. L. , O'Brien, C. A. & Manolagas, S. C. (2013). Estrogen receptor‐alpha signaling in osteoblast progenitors stimulates cortical bone accrual. Journal of Clinical Investigation, 123(1), 394–404. 10.1172/JCI65910 - DOI - PMC - PubMed
-
- Ambrosi, T. H. , Scialdone, A. , Graja, A. , Gohlke, S. , Jank, A. M. , Bocian, C. , Woelk, L. , Fan, H. , Logan, D. W. , Schürmann, A. , Saraiva, L. R. , & Schulz, T. J. (2017). Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell‐based hematopoietic and bone regeneration. Cell Stem Cell, 20(6), 771–784 e776. 10.1016/j.stem.2017.02.009 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

