Remote FEV1 Monitoring in Asthma Patients: A Pilot Study
- PMID: 33048470
- PMCID: PMC7993258
- DOI: 10.1111/cts.12901
Remote FEV1 Monitoring in Asthma Patients: A Pilot Study
Abstract
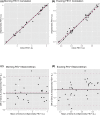
Forced expiratory volume in one second (FEV1 ) is a critical parameter for the assessment of lung function for both clinical care and research in patients with asthma. While asthma is defined by variable airflow obstruction, FEV1 is typically assessed during clinic visits. Mobile spirometry (mSpirometry) allows more frequent measurements of FEV1 , resulting in a more continuous assessment of lung function over time and its variability. Twelve patients with moderate asthma were recruited in a single-center study and were instructed to perform pulmonary function tests at home twice daily for 28 days and weekly in the clinic. Daily and mean subject compliances were summarized. The agreement between clinic and mobile FEV1 was assessed using correlation and Bland-Altman analyses. The test-retest reliability for clinic and mSpirometry was assessed by interclass correlation coefficient (ICC). Simulation was conducted to explore if mSpirometry could improve statistical power over clinic counterparts. The mean subject compliance with mSpirometry was 70% for twice-daily and 85% for at least once-daily. The mSpirometry FEV1 were highly correlated and agreed with clinic ones from the same morning (r = 0.993) and the same afternoon (r = 0.988) with smaller mean difference for the afternoon (0.0019 L) than morning (0.0126 L) measurements. The test-retest reliability of mobile (ICC = 0.932) and clinic (ICC = 0.942) spirometry were comparable. Our simulation analysis indicated greater power using dense mSpirometry than sparse clinic measurements. Overall, we have demonstrated good compliance for repeated at-home mSpirometry, high agreement and comparable test-retest reliability with clinic counterparts, greater statistical power, suggesting a potential for use in asthma clinical research.
© 2020 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
C.H., E.S.I., R.E., and G.B., are or were employees of Koneksa Health Inc., and may own company stock. N.J. has nothing to disclose. D.S. received sponsorship to attend and speak at international meetings, and honoraria for lecturing or attending advisory boards, from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Genentech, GlaxoSmithKline, Glenmark, Menarini, Mundipharma, Novartis, Peptinnovate, Pfizer, Pulmatrix, Teva, Therevance, and Verona. M.R. is an employee of Regeneron Pharmaceuticals.
Figures


References
-
- Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am. Rev. Respir. Dis. 136, 225–244 (1987). - PubMed
-
- Turner‐Warwick, M. Epidemiology of nocturnal asthma. Am. J. Med. 85, 6–8 (1988). - PubMed
-
- Sutherland, E.R. Nocturnal asthma. J. Allergy Clin. Immunol. 116, 1179–1186 (2005), quiz 1187. - PubMed
-
- Hansen, E.F. , Vestbo, J. , Phanareth, K. , Kok‐Jensen, A. & Dirksen, A. Peak flow as predictor of overall mortality in asthma and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 163, 690–693 (2001). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

