Chemoselective α-Sulfidation of Amides Using Sulfoxide Reagents
- PMID: 33048547
- PMCID: PMC7680396
- DOI: 10.1021/acs.orglett.0c03160
Chemoselective α-Sulfidation of Amides Using Sulfoxide Reagents
Abstract
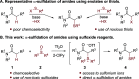
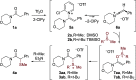
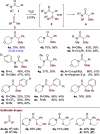
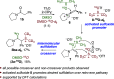
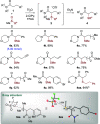
The direct α-sulfidation of tertiary amides using sulfoxide reagents under electrophilic amide activation conditions is described. Employing convenient and readily available reagents, selective functionalization takes place to generate isolable sulfonium ions en route to α-sulfide amides. Mechanistic studies identified activated sulfoxides as promoters of the desired transformation and enabled the extension of the methodology from benzylic to aliphatic amide substrates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Scott K. A.; Njardarson J. T. Analysis of US FDA-Approved Drugs Containing Sulfur Atoms. Top. Curr. Chem. 2018, 376, 1–34. 10.1007/978-3-030-25598-5_1. - DOI - PubMed
- Ilardi E. A.; Vitaku E.; Njardarson J. T. Data-Mining for Sulfur and Fluorine: An Evaluation of Pharmaceuticals to Reveal Opportunities for Drug Design and Discovery. J. Med. Chem. 2014, 57, 2832–2842. 10.1021/jm401375q. - DOI - PubMed
- Center for Drug Evaluation and Research . Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations, 40th ed.; U.S. Dept. of Health and Human Services: Rockville, MD, 2020.
-
-
For recent reviews on strategies for carbon–sulfur bond formation, see:
- Beletskaya I. P.; Ananikov V. P. Transition-Metal-Catalyzed C–S, C–Se, and C–Te Bond Formation Cross-Coupling and Atom-Economic Addition Reactions. Chem. Rev. 2011, 111, 1596–1636. 10.1021/cr100347k. - DOI - PubMed
- Eichman C. C.; Stambuli J. P. Transition Metal Catalyzed Synthesis of Aryl Sulfides. Molecules 2011, 16, 590–608. 10.3390/molecules16010590. - DOI - PMC - PubMed
- Chauhan P.; Mahajan S.; Enders D. Organocatalytic Carbon–Sulfur Bond-Forming Reactions. Chem. Rev. 2014, 114, 8807–8864. 10.1021/cr500235v. - DOI - PubMed
- Shen C.; Zhang P.; Sun Q.; Bai S.; Hor T. S. A.; Liu X. Recent advances in C–S bond formation via C–H bond functionalization and decarboxylation. Chem. Soc. Rev. 2015, 44, 291–314. 10.1039/C4CS00239C. - DOI - PubMed
- Wimmer A.; König B. Photocatalytic formation of carbon–sulfur bonds. Beilstein J. Org. Chem. 2018, 14, 54–83. 10.3762/bjoc.14.4. - DOI - PMC - PubMed
-
-
- Mukaiyama T.; Endo T.; Kojima Y.; Sato T. Selective Formation of Symmetrical Bissulfides. J. Am. Chem. Soc. 1972, 94, 7575–7577. 10.1021/ja00776a049. - DOI
- Zoretic P. A.; Soja P. Sulfenylation and Selenenylation of Lactams. J. Org. Chem. 1976, 41, 3587–3589. 10.1021/jo00884a022. - DOI
- Marzorati L.; Fejfar J. L.; Tormena C. F.; Vitta C. D. Kinetic Resolution of α-Bromophenylacetamides Using Quinine or Cinchona Alkaloid Salts. Tetrahedron: Asymmetry 2012, 23, 748–753. 10.1016/j.tetasy.2012.05.009. - DOI
- Zou L.-H.; Priebbenow D. L.; Wang L.; Mottweiler J.; Bolm C. Copper-Catalyzed Synthesis of α-Thioaryl Carbonyl Compounds Through S–S and C–C Bond Cleavage. Adv. Synth. Catal. 2013, 355, 2558–2563. 10.1002/adsc.201300566. - DOI
- Prasad C. D.; Sattar M.; Kumar S. Transition-Metal-Free Selective Oxidative C(sp3)–S/Se Coupling of Oxindoles, Tetralone, and Arylacetamides: Synthesis of Unsymmetrical Organochalcogenides. Org. Lett. 2017, 19, 774–777. 10.1021/acs.orglett.6b03735. - DOI - PubMed
- Jiang Y.; Deng J.-D.; Wang H.-D.; Zou J.-X.; Wang Y.-Q.; Chen J.-H.; Zhu L.-Q.; Zhang H.-H.; Peng X.; Wang Z. Direct access to α-sulfenylated amides/esters via sequential oxidative sulfenylation and C–C bond cleavage of 3-oxobutyric amides/esters. Chem. Commun. 2018, 54, 802–805. 10.1039/C7CC09026A. - DOI - PubMed
- Gonçalves C. R.; Lemmerer M.; Teskey C. J.; Adler P.; Kaiser D.; Maryasin B.; González L.; Maulide N. Unified Approach to the Chemoselective α-Functionalization of Amides with Heteroatom Nucleophiles. J. Am. Chem. Soc. 2019, 141, 18437–18443. 10.1021/jacs.9b06956. - DOI - PMC - PubMed
- Liu C.; Li Z.; Weng Z.; Fang X.; Zhao F.; Tang K.; Chen J.; Ma W. Transition-Metal-Free Selective C(sp3)–H Thiolation of Arylacetamides with Substituted Benzenethiols, Aryl Sulfenylchlorides and Diaryl Disulfides. Asian J. Org. Chem. 2020, 9, 668–672. 10.1002/ajoc.202000083. - DOI
-
- Movassaghi M.; Hill M. D. Synthesis of Substituted Pyridine Derivatives via the Ruthenium-Catalyzed Cycloisomerization of 3-Azadienynes. J. Am. Chem. Soc. 2006, 128, 4592–4593. 10.1021/ja060626a. - DOI - PubMed
- Movassaghi M.; Hill M. D. Single-Step Synthesis of Pyrimidine Derivatives. J. Am. Chem. Soc. 2006, 128, 14254–14255. 10.1021/ja066405m. - DOI - PubMed
- Movassaghi M.; Hill M. D.; Ahmad O. K. Direct Synthesis of Pyridine Derivatives. J. Am. Chem. Soc. 2007, 129, 10096–10097. 10.1021/ja073912a. - DOI - PubMed
- Movassaghi M.; Hill M. D. A Versatile Cyclodehydration Reaction for the Synthesis of Isoquinoline and β-Carboline Derivatives. Org. Lett. 2008, 10, 3485–3488. 10.1021/ol801264u. - DOI - PMC - PubMed
- Medley J. W.; Movassaghi M. Direct Dehydrative N-Pyridinylation of Amides. J. Org. Chem. 2009, 74, 1341–1344. 10.1021/jo802355d. - DOI - PubMed
- Ahmad O. K.; Hill M. D.; Movassaghi M. Synthesis of Densely Substituted Pyrimidine Derivatives. J. Org. Chem. 2009, 74, 8460–8463. 10.1021/jo9017149. - DOI - PubMed
- Medley J. W.; Movassaghi M. Synthesis of Spirocyclic Indolines by Interruption of the Bischler–Napieralski Reaction. Org. Lett. 2013, 15, 3614–3617. 10.1021/ol401465y. - DOI - PMC - PubMed
- White K. L.; Mewald M.; Movassaghi M. Direct Observation of Intermediates Involved in the Interruption of the Bischler–Napieralski Reaction. J. Org. Chem. 2015, 80, 7403–7411. 10.1021/acs.joc.5b01023. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

