Facemask against viral respiratory infections among Hajj pilgrims: A challenging cluster-randomized trial
- PMID: 33048964
- PMCID: PMC7553311
- DOI: 10.1371/journal.pone.0240287
Facemask against viral respiratory infections among Hajj pilgrims: A challenging cluster-randomized trial
Abstract
Background: In this large-scale cluster-randomized controlled trial (cRCT) we sought to assess the effectiveness of facemasks against viral respiratory infections.
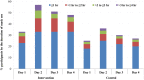
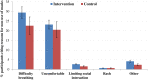
Methods and results: Over three consecutive Hajj seasons (2013, 2014, 2015) pilgrims' tents in Makkah were allocated to 'facemask' or 'no facemask' group. Fifty facemasks were offered to participants in intervention tents, to be worn over four days, and none were offered to participants in control tents. All participants recorded facemask use and respiratory symptoms in health diaries. Nasal swabs were collected from the symptomatic for virus detection by reverse transcription polymerase chain reaction. Clinical symptoms and laboratory results were analyzed by 'intention- to-treat' and 'per-protocol'. A total of 7687 adult participants from 318 tents were randomized: 3864 from 149 tents to the intervention group, and 3823 from 169 tents to the control group. Participants were aged 18 to 95 (median 34, mean 37) years, with a male to female ratio of 1:1.2. Overall, respiratory viruses were detected in 277 of 650 (43%) nasal/pharyngeal swabs collected from symptomatic pilgrims. Common viruses were rhinovirus (35.1%), influenza (4.5%) and parainfluenza (1.7%). In the intervention arm, respectively 954 (24.7%) and 1842 (47.7%) participants used facemasks daily and intermittently, while in the control arm, respectively 546 (14.3%) and 1334 (34.9%) used facemasks daily and intermittently. By intention-to-treat analysis, facemask use did not seem to be effective against laboratory-confirmed viral respiratory infections (odds ratio [OR], 1.4; 95% confidence interval [CI], 0.9 to 2.1, p = 0.18) nor against clinical respiratory infection (OR, 1.1; 95% CI, 0.9 to 1.4, p = 0.40). Similarly, in a per-protocol analysis, facemask use did not seem to be effective against laboratory-confirmed viral respiratory infections (OR 1.2, 95% CI 0.9-1.7, p = 0.26) nor against clinical respiratory infection (OR 1.3, 95% CI 1.0-1.8, p = 0.06).
Conclusion: This trial was unable to provide conclusive evidence on facemask efficacy against viral respiratory infections most likely due to poor adherence to protocol.
Conflict of interest statement
Professor Robert Booy has received funding from Baxter, CSL, GSK, Merck, Novartis, Pfizer, Roche, Romark and Sanofi Pasteur for conducting research other than this, travel to conferences or consultancy work; all funding received is directed to research accounts at The Children’s Hospital at Westmead. Dr Harunor Rashid has received fees from Pfizer, Sanofi Pasteur and Novartis for consulting or serving on an advisory board. The other authors have no competing interests to declare.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

