IL-27 signalling regulates glycolysis in Th1 cells to limit immunopathology during infection
- PMID: 33049000
- PMCID: PMC7584222
- DOI: 10.1371/journal.ppat.1008994
IL-27 signalling regulates glycolysis in Th1 cells to limit immunopathology during infection
Abstract
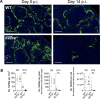
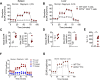
Inflammation is critical for controlling pathogens, but also responsible for symptoms of infectious diseases. IL-27 is an important regulator of inflammation and can limit development of IFNγ-producing Tbet+ CD4+ T (Th1) cells. IL-27 is thought to do this by stimulating IL-10 production by CD4+ T cells, but the underlying mechanisms of these immunoregulatory pathways are not clear. Here we studied the role of IL-27 signalling in experimental visceral leishmaniasis (VL) caused by infection of C57BL/6 mice with the human pathogen Leishmania donovani. We found IL-27 signalling was critical for the development of IL-10-producing Th1 (Tr1) cells during infection. Furthermore, in the absence of IL-27 signalling, there was improved control of parasite growth, but accelerated splenic pathology characterised by the loss of marginal zone macrophages. Critically, we discovered that IL-27 signalling limited glycolysis in Th1 cells during infection that in turn attenuated inflammation. Furthermore, the modulation of glycolysis in the absence of IL-27 signalling restricted tissue pathology without compromising anti-parasitic immunity. Together, these findings identify a novel mechanism by which IL-27 mediates immune regulation during disease by regulating cellular metabolism.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Herwaldt BL. Leishmaniasis. Lancet (London, England). 1999;354(9185):1191–9. - PubMed
-
- Burza S, Croft SL, Boelaert M. Leishmaniasis. The Lancet. 2018;392(10151):951–70. - PubMed
-
- Rosas LE, Satoskar AA, Roth KM, Keiser TL, Barbi J, Hunter C, et al. Interleukin-27R (WSX-1/T-Cell Cytokine Receptor) Gene-Deficient Mice Display Enhanced Resistance to Leishmania donovani Infection but Develop Severe Liver Immunopathology. The American Journal of Pathology. 2006;168(1):158–69. 10.2353/ajpath.2006.050013 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

