Laboratory variability in the diagnosis of type 2 VWD variants
- PMID: 33049112
- PMCID: PMC7790985
- DOI: 10.1111/jth.15129
Laboratory variability in the diagnosis of type 2 VWD variants
Abstract
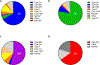
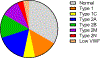
Essentials Patients with von Willebrand disease were enrolled in our study. Type 2 VWD diagnoses were based on original test results. Repeat evaluation resulted in many patients receiving a different type 2 diagnosis. Some genetic variants were particularly likely to move type 2 subcategories. ABSTRACT: Introduction Type 2 von Willebrand disease (VWD) refers to patients with a qualitative defect in von Willebrand factor. Accurate diagnosis of type 2 VWD subtypes can be challenging. Aim of the study To compare the historical diagnosis of type 2 VWD with current laboratory testing. Methods Subjects were enrolled in the Zimmerman Program either because of a preexisting diagnosis of VWD (retrospective cohort) or from evaluation for bleeding symptoms or suspected VWD (prospective cohort). Original diagnosis was assigned by the local center and central diagnosis was based on central laboratory testing. Results Two hundred and seventeen index cases in the retrospective cohort and 35 subjects in the prospective cohort carried a local diagnosis of type 2 VWD (29% and 6% of enrolled index cases, respectively). In the retrospective cohort, the diagnosis was confirmed in 66% of cases with a preexisting diagnosis of 2A, 77% 2B, 54% 2M, and 72% 2N. In the prospective cohort, 31% were confirmed 2A, 60% 2B, 23% 2M, and 100% 2N. Several genetic variants were repeatedly implicated in subjects with changed diagnosis: p.M1304R, p.R1315C, p.R1374C, and p.R1374H. Conclusions Both the prospective and retrospective cohorts demonstrated consistent variation in subjects whose diagnosis changed between 2A, 2B, and 2M. The importance of accurately diagnosing type 2 VWD may be most significant in the 2B subtype given potential concerns with the use of desmopressin in type 2B VWD. Some genetic variants appear in multiple types of VWD, making specific diagnoses challenging.
Keywords: Von Willebrand disease; Von Willebrand factor; genetic; medical laboratory science; polymorphism; ristocetin.
© 2020 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
Disclosure of Conflicts of Interest:
RRM holds a patent assigned to the Versiti Blood Center of Wisconsin for a VWF platelet binding assay. The remaining authors report no conflicts of interest.
Figures
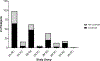
References
-
- Bowman M, Hopman WM, Rapson D, Lillicrap D, James P. The prevalence of symptomatic von Willebrand disease in primary care practice. J Thromb Haemost. 2010;8:213–216. - PubMed
-
- Nichols WL, Hultin MB, James AH, Manco-Johnson MJ, Montgomery RR, Ortel TL, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia. 2008;14:171–232. - PubMed
-
- Sadler JE, Budde U, Eikenboom JC, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost. 2006;4:2103–2114. - PubMed
-
- Budde U, Schneppenheim R, Eikenboom J, Goodeve A, Will K, Drewke E, et al. Detailed von Willebrand factor multimer analysis in patients with von Willebrand disease in the European study, molecular and clinical markers for the diagnosis and management of type 1 von Willebrand disease (MCMDM-1VWD). J Thromb Haemost. 2008;6:762–771. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

