The heterogeneous morphology of networked collagen in distal colon and rectum of mice quantified via nonlinear microscopy
- PMID: 33049619
- PMCID: PMC7725919
- DOI: 10.1016/j.jmbbm.2020.104116
The heterogeneous morphology of networked collagen in distal colon and rectum of mice quantified via nonlinear microscopy
Abstract
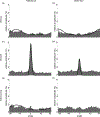
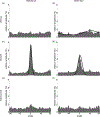
Visceral pain from the distal colon and rectum (colorectum) is a major complaint of patients with irritable bowel syndrome. Mechanotransduction of colorectal distension/stretch appears to play a critical role in visceral nociception, and further understanding requires improved knowledge of the micromechanical environments at different sub-layers of the colorectum. In this study, we conducted nonlinear imaging via second harmonic generation to quantify the thickness of each distinct through-thickness layer of the colorectum, as well as the principal orientations, corresponding dispersions in orientations, and the distributions of diameters of collagen fibers within each of these layers. From C57BL/6 mice of both sexes (8-16 weeks of age, 25-35 g), we dissected the distal 30 mm of the large bowel including the colorectum, divided these into three even segments, and harvested specimens (~8 × 8 mm2) from each segment. We stretched the specimens either by colorectal distension to 20 mmHg (reference) or 80 mmHg (deformed) or by biaxial stretch to 10 mN (reference) or 80 mN (deformed), and fixed them with 4% paraformaldehyde. We then conducted SHG imaging through the wall thickness and analyzed post-hoc using custom-built software to quantify the orientations of collagen fibers in all distinct layers. We also quantified the thickness of each layer of the colorectum, and the corresponding distributions of collagen density and diameters of fibers. We found collagen concentrated in the submucosal layer. The average diameter of collagen fibers was greatest in the submucosal layer, followed by the serosal and muscular layers. Collagen fibers aligned with muscle fibers in the two muscular layers, whereas their orientation varied greatly with location in the serosal layer. In colonic segments, thick collagen fibers in the submucosa presented two major orientations aligned approximately ±30° to the axial direction, and form a patterned network. Our results indicate the submucosa is likely the principal passive load-bearing structure of the colorectum. In addition, afferent endings in those collagen-rich regions present likely candidates of colorectal nociceptors to encode noxious distension/stretch.
Keywords: Biaxial extension test; Colorectum; Confocal microscopy; Mechanotransduction; Submucosa; Visceral pain.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of Interest
We have no conflicts of interest to report.
Figures





Similar articles
-
The Macro- and Micro-Mechanics of the Colon and Rectum I: Experimental Evidence.Bioengineering (Basel). 2020 Oct 19;7(4):130. doi: 10.3390/bioengineering7040130. Bioengineering (Basel). 2020. PMID: 33086503 Free PMC article. Review.
-
Load-bearing function of the colorectal submucosa and its relevance to visceral nociception elicited by mechanical stretch.Am J Physiol Gastrointest Liver Physiol. 2019 Sep 1;317(3):G349-G358. doi: 10.1152/ajpgi.00127.2019. Epub 2019 Jul 3. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 31268771 Free PMC article.
-
Differential biomechanical properties of mouse distal colon and rectum innervated by the splanchnic and pelvic afferents.Am J Physiol Gastrointest Liver Physiol. 2019 Apr 1;316(4):G473-G481. doi: 10.1152/ajpgi.00324.2018. Epub 2019 Jan 31. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 30702901 Free PMC article.
-
Computational Modeling of Mouse Colorectum Capturing Longitudinal and Through-thickness Biomechanical Heterogeneity.J Mech Behav Biomed Mater. 2021 Jan;113:104127. doi: 10.1016/j.jmbbm.2020.104127. Epub 2020 Oct 10. J Mech Behav Biomed Mater. 2021. PMID: 33125950 Free PMC article.
-
Visceral pain from colon and rectum: the mechanotransduction and biomechanics.J Neural Transm (Vienna). 2020 Apr;127(4):415-429. doi: 10.1007/s00702-019-02088-8. Epub 2019 Oct 9. J Neural Transm (Vienna). 2020. PMID: 31598778 Free PMC article. Review.
Cited by
-
The Macro- and Micro-Mechanics of the Colon and Rectum I: Experimental Evidence.Bioengineering (Basel). 2020 Oct 19;7(4):130. doi: 10.3390/bioengineering7040130. Bioengineering (Basel). 2020. PMID: 33086503 Free PMC article. Review.
-
Optical clearing reveals TNBS-induced morphological changes of VGLUT2-positive nerve fibers in mouse colorectum.Am J Physiol Gastrointest Liver Physiol. 2021 Apr 1;320(4):G644-G657. doi: 10.1152/ajpgi.00363.2020. Epub 2021 Feb 3. Am J Physiol Gastrointest Liver Physiol. 2021. PMID: 33533318 Free PMC article.
-
Morphological and Functional Colonic Defects Caused by a Mutated Thyroid Hormone Receptor α.Thyroid. 2023 Feb;33(2):239-250. doi: 10.1089/thy.2022.0336. Epub 2022 Nov 3. Thyroid. 2023. PMID: 36103385 Free PMC article.
-
Layer-Specific Damage Modeling of Porcine Large Intestine under Biaxial Tension.Bioengineering (Basel). 2022 Oct 6;9(10):528. doi: 10.3390/bioengineering9100528. Bioengineering (Basel). 2022. PMID: 36290495 Free PMC article.
-
Predicting the micromechanics of embedded nerve fibers using a novel three-layered model of mouse distal colon and rectum.J Mech Behav Biomed Mater. 2022 Mar;127:105083. doi: 10.1016/j.jmbbm.2022.105083. Epub 2022 Jan 20. J Mech Behav Biomed Mater. 2022. PMID: 35093713 Free PMC article.
References
-
- Arutyunov GP, Kostyukevich OI, Serov RA, Rylova NV, Bylova NA, 2008. Collagen accumulation and dysfunctional mucosal barrier of the small intestine in patients with chronic heart failure. International journal of cardiology 125, 240–245. - PubMed
-
- Brierley SM, Jones RC 3rd, Gebhart GF, Blackshaw LA, 2004. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127, 166–178. - PubMed
-
- Brierley SM, Jones RC III, Xu L, Gebhart GF, Blackshaw LA, 2005b. Activation of splanchnic and pelvic colonic afferents by bradykinin in mice. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 17, 854–862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

