Initial In Vitro and In Vivo Evaluation of a Novel CCK2R Targeting Peptide Analog Labeled with Lutetium-177
- PMID: 33049999
- PMCID: PMC7583830
- DOI: 10.3390/molecules25194585
Initial In Vitro and In Vivo Evaluation of a Novel CCK2R Targeting Peptide Analog Labeled with Lutetium-177
Abstract
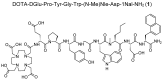
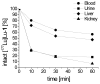
Targeting of cholecystokinin-2 receptor (CCK2R) expressing tumors using radiolabeled minigastrin (MG) analogs is hampered by rapid digestion of the linear peptide in vivo. In this study, a new MG analog stabilized against enzymatic degradation was investigated in preclinical studies to characterize the metabolites formed in vivo. The new MG analog DOTA-DGlu-Pro-Tyr-Gly-Trp-(N-Me)Nle-Asp-1Nal-NH2 comprising site-specific amino acid substitutions in position 2, 6 and 8 and different possible metabolites thereof were synthesized. The receptor interaction of the peptide and selected metabolites was evaluated in a CCK2R-expressing cell line. The enzymatic stability of the 177Lu-labeled peptide analog was evaluated in vitro in different media as well as in BALB/c mice up to 1 h after injection and the metabolites were identified based on radio-HPLC analysis. The new radiopeptide showed a highly increased stability in vivo with >56% intact radiopeptide in the blood of BALB/c mice 1 h after injection. High CCK2R affinity and cell uptake was confirmed only for the intact peptide, whereas enzymatic cleavage within the receptor specific C-terminal amino acid sequence resulted in complete loss of affinity and cell uptake. A favorable biodistribution profile was observed in BALB/c mice with low background activity, preferential renal excretion and prolonged uptake in CCK2R-expressing tissues. The novel stabilized MG analog shows high potential for diagnostic and therapeutic use. The radiometabolites characterized give new insights into the enzymatic degradation in vivo.
Keywords: cholecystokinin-2 receptor; lutetium-177; minigastrin; molecular imaging; targeted radiotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Site-specific stabilization of minigastrin analogs against enzymatic degradation for enhanced cholecystokinin-2 receptor targeting.Theranostics. 2018 Apr 16;8(11):2896-2908. doi: 10.7150/thno.24378. eCollection 2018. Theranostics. 2018. PMID: 29896292 Free PMC article.
-
Targeting of the Cholecystokinin-2 Receptor with the Minigastrin Analog 177Lu-DOTA-PP-F11N: Does the Use of Protease Inhibitors Further Improve In Vivo Distribution?J Nucl Med. 2019 Mar;60(3):393-399. doi: 10.2967/jnumed.118.207845. Epub 2018 Jul 12. J Nucl Med. 2019. PMID: 30002107
-
DOTA-MGS5, a New Cholecystokinin-2 Receptor-Targeting Peptide Analog with an Optimized Targeting Profile for Theranostic Use.J Nucl Med. 2019 Jul;60(7):1010-1016. doi: 10.2967/jnumed.118.221283. Epub 2018 Dec 7. J Nucl Med. 2019. PMID: 30530828 Free PMC article.
-
111In/68Ga-Labeled DOTA-conjugated cyclo[γ-d-Glu-Ala-Tyr-d-Lys]-Trp-Met-Asp-Phe-NH2 (cyclo-MG1), a minigastrin analog.2012 May 17 [updated 2012 Jul 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 May 17 [updated 2012 Jul 12]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 22787691 Free Books & Documents. Review.
-
111In/68Ga-Labeled DOTA conjugated cyclo[γ-d-Glu-Ala-Tyr-d-Lys]-Trp-Nle-Asp-Phe-NH2 (cyclo-MG2), a minigastrin analog.2012 May 17 [updated 2012 Jul 26]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 May 17 [updated 2012 Jul 26]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 22855967 Free Books & Documents. Review.
Cited by
-
[68Ga]Ga-NODAGA-TriGalactan, a low molecular weight tracer for the non-invasive imaging of the functional liver reserve.EJNMMI Radiopharm Chem. 2024 May 15;9(1):41. doi: 10.1186/s41181-024-00271-1. EJNMMI Radiopharm Chem. 2024. PMID: 38750246 Free PMC article.
-
Investigation of the structure-activity relationship at the N-terminal part of minigastrin analogs.EJNMMI Res. 2023 Jul 8;13(1):65. doi: 10.1186/s13550-023-01016-y. EJNMMI Res. 2023. PMID: 37421545 Free PMC article.
-
Effect of N-Terminal Peptide Modifications on In Vitro and In Vivo Properties of 177Lu-Labeled Peptide Analogs Targeting CCK2R.Pharmaceutics. 2023 Feb 28;15(3):796. doi: 10.3390/pharmaceutics15030796. Pharmaceutics. 2023. PMID: 36986657 Free PMC article.
-
68Ga-Labeled Glycopeptides as Effective Tools for Liver Function Imaging.Mol Pharm. 2025 Mar 3;22(3):1677-1685. doi: 10.1021/acs.molpharmaceut.4c01453. Epub 2025 Feb 17. Mol Pharm. 2025. PMID: 39960224 Free PMC article.
-
Development of INER-PP-F11N as the Peptide-Radionuclide Conjugate Drug Against CCK2 Receptor-Overexpressing Tumors.Int J Mol Sci. 2025 Jul 8;26(14):6565. doi: 10.3390/ijms26146565. Int J Mol Sci. 2025. PMID: 40724817 Free PMC article.
References
-
- Bison S.M., Konijnenberg M.W., Melis M., Pool S.E., Bernsen M.R., Teunissen J.J., Kwekkeboom D.J., de Jong M. Peptide receptor radionuclide therapy using radiolabeled somatostatin analogs: Focus on future developments. Clin. Transl. Imaging. 2014;2:55–66. doi: 10.1007/s40336-014-0054-2. - DOI - PMC - PubMed
-
- Lutathera EMA. [(accessed on 21 September 2020)]; Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/lutathera.
-
- Lutathera FDA. [(accessed on 21 September 2020)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/208700Orig1s000T....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

