Syncytia in Fungi
- PMID: 33050028
- PMCID: PMC7600787
- DOI: 10.3390/cells9102255
Syncytia in Fungi
Abstract
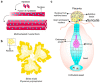
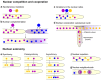
Filamentous fungi typically grow as interconnected multinucleate syncytia that can be microscopic to many hectares in size. Mechanistic details and rules that govern the formation and function of these multinucleate syncytia are largely unexplored, including details on syncytial morphology and the regulatory controls of cellular and molecular processes. Recent discoveries have revealed various adaptations that enable fungal syncytia to accomplish coordinated behaviors, including cell growth, nuclear division, secretion, communication, and adaptation of the hyphal network for mixing nuclear and cytoplasmic organelles. In this review, we highlight recent studies using advanced technologies to define rules that govern organizing principles of hyphal and colony differentiation, including various aspects of nuclear and mitochondrial cooperation versus competition. We place these findings into context with previous foundational literature and present still unanswered questions on mechanistic aspects, function, and morphological diversity of fungal syncytia across the fungal kingdom.
Keywords: filamentous fungi; heterokaryon; morphology; nucleus; syncytia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Abmayr S.M., Zhuang S., Geisbrecht E.R. Myoblast fusion in Drosophila. Methods Mol. Biol. 2008;475:75–97. - PubMed

