The Impact of Mitochondrial Deficiencies in Neuromuscular Diseases
- PMID: 33050147
- PMCID: PMC7600520
- DOI: 10.3390/antiox9100964
The Impact of Mitochondrial Deficiencies in Neuromuscular Diseases
Abstract
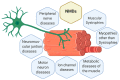
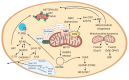
Neuromuscular diseases (NMDs) are a heterogeneous group of acquired or inherited rare disorders caused by injury or dysfunction of the anterior horn cells of the spinal cord (lower motor neurons), peripheral nerves, neuromuscular junctions, or skeletal muscles leading to muscle weakness and waste. Unfortunately, most of them entail serious or even fatal consequences. The prevalence rates among NMDs range between 1 and 10 per 100,000 population, but their rarity and diversity pose difficulties for healthcare and research. Some molecular hallmarks are being explored to elucidate the mechanisms triggering disease, to set the path for further advances. In fact, in the present review we outline the metabolic alterations of NMDs, mainly focusing on the role of mitochondria. The aim of the review is to discuss the mechanisms underlying energy production, oxidative stress generation, cell signaling, autophagy, and inflammation triggered or conditioned by the mitochondria. Briefly, increased levels of inflammation have been linked to reactive oxygen species (ROS) accumulation, which is key in mitochondrial genomic instability and mitochondrial respiratory chain (MRC) dysfunction. ROS burst, impaired autophagy, and increased inflammation are observed in many NMDs. Increasing knowledge of the etiology of NMDs will help to develop better diagnosis and treatments, eventually reducing the health and economic burden of NMDs for patients and healthcare systems.
Keywords: damage-associated molecular patterns (DAMPs); mitochondrial respiratory chain (MRC); mitophagy; neuromuscular diseases (NMDs); oxidative stress; reactive oxygen species (ROS).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Diagnosis—European Reference Network—EURO-NMD. [(accessed on 22 April 2020)]; Available online: https://ern-euro-nmd.eu/
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

